Products may be shipped at a different temperature than the recommended long-term storage temperature. If the product quality is sensitive to short-term exposure to conditions other than the recommended long-term storage, it will be shipped on wet or dry-ice. If the product quality is NOT affected by short-term exposure to conditions other than the recommended long-term storage, it will be shipped at ambient temperature. As shipping routes are configured for minimum transit times, shipping at ambient temperature helps control shipping costs for our customers. For more information, please refer to the Storage and Transport Conditions document: https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/marketing/global/documents/316/622/storage-transport-conditions-mk.pdf
230707
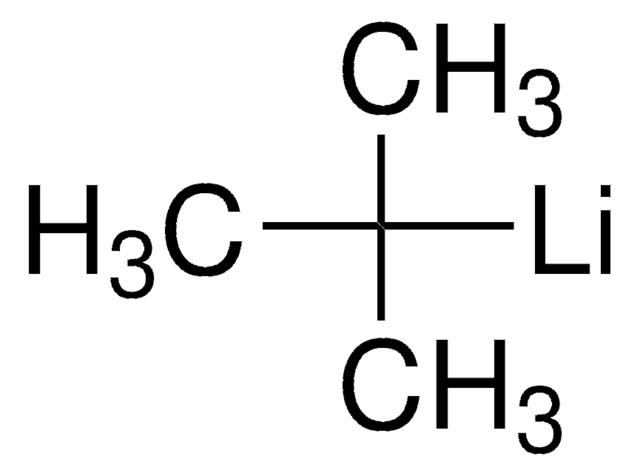
n-Butil-litio solution
2.5 M in hexanes
Sinónimos:
n-BuLi, Butil litio, Butil-litio solution, Litio-1-butanida
About This Item
Productos recomendados
form
liquid
Quality Level
concentration
2.5 M in hexanes
density
0.693 g/mL at 25 °C
storage temp.
2-8°C
SMILES string
[Li]CCCC
InChI
1S/C4H9.Li/c1-3-4-2;/h1,3-4H2,2H3;
InChI key
MZRVEZGGRBJDDB-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Application
El producto también se utiliza en las siguientes reacciones:
- Reacciones de reordenación aniónica
- Reacciones de intercambio de metal-halógeno y de transmetalización
- Reacciones de eliminación
- Reacción de reordenación de [1,2]- y [1,4]-Wittig
- Reacción de transposición aniónica de homo-Fries
- Carbolitiación asimétrica
Packaging
Legal Information
Optional
related product
signalword
Danger
Hazard Classifications
Aquatic Chronic 2 - Asp. Tox. 1 - Eye Dam. 1 - Flam. Liq. 2 - Pyr. Liq. 1 - Repr. 2 - Skin Corr. 1B - STOT SE 3 - Water-react 1
target_organs
Central nervous system
supp_hazards
Storage Class
4.2 - Pyrophoric and self-heating hazardous materials
wgk_germany
WGK 3
flash_point_f
-7.6 °F - closed cup
flash_point_c
-22 °C - closed cup
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Transformative reagents enable selective conversions within molecules containing sensitive functionalities under mild reactions.
Transformative reagents enable selective conversions within molecules containing sensitive functionalities under mild reactions.
Transformative reagents enable selective conversions within molecules containing sensitive functionalities under mild reactions.
Transformative reagents enable selective conversions within molecules containing sensitive functionalities under mild reactions.
-
How is shipping temperature determined? And how is it related to the product storage temperature?
1 answer-
Helpful?
-
-
How can I determine the shelf life / expiration / retest date of this product?
1 answer-
If this product has an expiration or retest date, it will be shown on the Certificate of Analysis (COA, CofA). If there is no retest or expiration date listed on the product's COA, we do not have suitable stability data to determine a shelf life. For these products, the only date on the COA will be the release date; a retest, expiration, or use-by-date will not be displayed.
For all products, we recommend handling per defined conditions as printed in our product literature and website product descriptions. We recommend that products should be routinely inspected by customers to ensure they perform as expected.
For products without retest or expiration dates, our standard warranty of 1 year from the date of shipment is applicable.
For more information, please refer to the Product Dating Information document: https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/marketing/global/documents/449/386/product-dating-information-mk.pdfHelpful?
-
-
Why is there a plastic layer around the glass bottle?
1 answer-
The plastic (PVC) coating on the glass bottle is an added safety measure in high-wear situations. The coating also provides a degree of shatter resistance to the glass bottle, as well a an improved gripping surface when compared to non-coated glass.
Helpful?
-
-
What are common uses for Product 230707, Butyllithium solution?
1 answer-
Butyllithium is widely use as a polymerization initiator in the production of elastomers such as polybutadiene or styrene-butadiene-styrene (SBS). It is also broadly employed as a strong base (superbase) in organic synthesis, both industrially and in the laboratory.
Helpful?
-
-
How are the 8 L and 18 L unit sizes of Product 230707, Butyllithium solution, packaged?
1 answer-
They are packaged in our Kilo-Lab™ cylinder.
Helpful?
-
-
What is the Department of Transportation shipping information for this product?
1 answer-
Transportation information can be found in Section 14 of the product's (M)SDS.To access the shipping information for this material, use the link on the product detail page for the product.
Helpful?
-
-
How are the 100 ml and 800 ml unit sizes of Product 230707, Butyllithium solution,packaged?
1 answer-
They are packaged in our Sure-Seal™ bottle, which have a crown cap with a PTFE/elastomer liner crimped into place.
Helpful?
-
-
Is it necessary to handle Product 230707, Butyllithium solution, under inert atmosphere?
1 answer-
This product ignites on exposure to air and should be handled under inert atmosphere such as under nitrogen.
Helpful?
-
-
What should I do if precipitate is present in Product 230707, Butyllithium solution?
1 answer-
Haziness may form upon storage but does not affect the specification. If precipitation is present, then gently mix precipitate back into solution under an inert atmosphere.
Helpful?
-
Active Filters
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico