157449
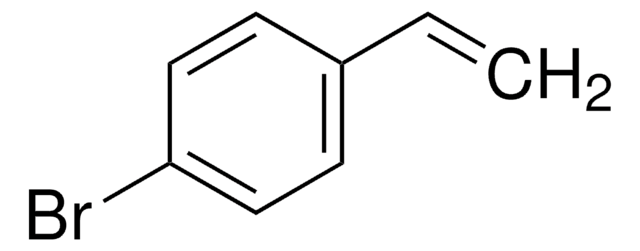
β-Bromostyrene
97%
Sinónimos:
beta-Bromostyrene, 1-Bromo-2-phenylethylene
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
C6H5CH=CHBr
Número de CAS:
Peso molecular:
183.05
Beilstein/REAXYS Number:
2038495
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
assay
97%
form
liquid
refractive index
n20/D 1.607 (lit.)
bp
110-112 °C/20 mmHg (lit.)
mp
7 °C (lit.)
density
1.427 g/mL at 25 °C (lit.)
functional group
bromo
storage temp.
2-8°C
SMILES string
Br\C=C\c1ccccc1
InChI
1S/C8H7Br/c9-7-6-8-4-2-1-3-5-8/h1-7H/b7-6+
InChI key
YMOONIIMQBGTDU-VOTSOKGWSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
β-Bromostyrene is an α,β-unsaturated aromatic halide. It can be synthesized by catalytic Hunsdiecker reaction (CHR) of cinnamic acid. It is commonly utilized as a precursor for preparing substituted alkenes, corresponding acetylenes and also in the total synthesis of natural compounds and antibiotics.
Application
β-Bromostyrene was used in the one-pot method for the preparation of cinnamonitriles. It was also used in the preparation of pure ethyl Z- and E-α,α-difluoro-4-phenyl-3-butenoate and 1,3-diphenyl-1-butene. It may be used in the synthesis of β-tert-butylstyrene via cross coupling reaction with tert-butylmagnesium chloride in the presence of dichloro[1,1′-bis(diphenylphosphino)ferrocene]nickel(II) catalyst. It may also be used to prepare γ-but-2-enolactone by reacting with nickel carbonyl in the presence of alkynes.
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Cross-Coupling of Tertiary Alkyl Grignard Reagents with β-Bromostyrene Catalyzed by Dichloro[1,1'-bis(diphenylphosphino)ferrocene]nickel (II).
Hayashi T, et al.
Chemistry Letters (Jpn), 9(6), 767-768 (1980)
The reaction of ?-bromostyrene with nickel carbonyl in the presence of alkynes.
Ryang M, et al.
Journal of Organometallic Chemistry, 46(2), 375-377 (1972)
Enantioselective Preparation of C2-Symmetrical Ferrocenyl Ligands for Asymmetric Catalysis.
Schwink L and Knochel P.
Chemistry (Weinheim An Der Bergstrasse, Germany), 4(5), 950-968 (1998)
Catalytic Hunsdiecker Reaction and One-Pot Catalytic Hunsdiecker-Heck Strategy: Synthesis of α, β-Unsaturated Aromatic Halides, α-(Dihalomethyl) benzenemethanols, 5-Aryl-2, 4-pentadienoic acids, Dienoates and Dienamides.
Naskar D and Roy S.
Tetrahedron, 56(10), 1369-1377 (2000)
Practical one-pot conversion of aryl bromides and ?-bromostyrenes into aromatic nitriles and cinnamonitriles.
Ishii G, et al.
Tetrahedron, 69(5), 1462-1469 (2013)
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico