129593
Malonamide
97%
Sinónimos:
Malonodiamide
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
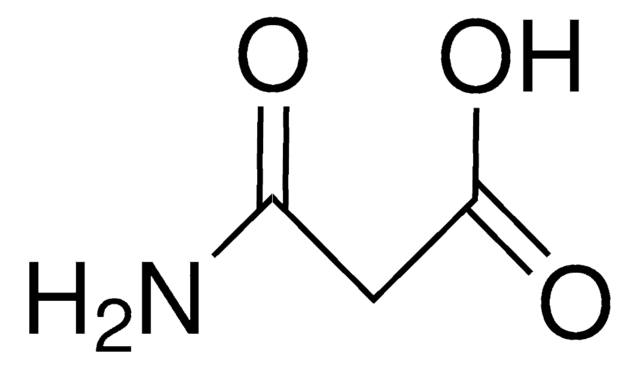
Fórmula lineal:
CH2(CONH2)2
Número de CAS:
Peso molecular:
102.09
Beilstein/REAXYS Number:
1751401
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
97%
form
solid
mp
172-175 °C (lit.)
fluorescence
λex 367 nm; λem 445 nm (α-keto acid adduct)
SMILES string
NC(=O)CC(N)=O
InChI
1S/C3H6N2O2/c4-2(6)1-3(5)7/h1H2,(H2,4,6)(H2,5,7)
InChI key
WRIRWRKPLXCTFD-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
The malonamide derivatives are obtained by the one-pot, five-component condensation reaction of isocyanide, Meldrum′s acid, arylidene malononitrile, and two amine molecules in CH2Cl2.
Application
The malonamide-based ionic liquid extractant was used in the extraction of europium(iii) and other trivalent rare-earth ions from nitric acid medium.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 2
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Ana G Neo et al.
Molecular diversity, 15(2), 529-539 (2010-09-03)
A general synthesis of 1,3-dicarbonylic compounds using multicomponent reactions of isocyanides is described. The process involves a Passerini three-component condensation of glyoxal derivatives, isocyanides and acetic acid, followed by metal mediated reductive or solvolytic removal of the acid component. Noteworthy
M J Barlow et al.
Solid state nuclear magnetic resonance, 1(4), 197-204 (1992-11-01)
Methyl tunnel frequencies, measured at 4 K, are found to be 455 +/- 8 kHz in methyl malonamide and 496 +/- 8 kHz in methyl ethyl ketone. The first is unaffected by deuteration of the amide groups. Measurements of the
M M Schiavoni et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 56A(8), 1533-1541 (2000-07-25)
The conformational and tautomeric compositions of malonamide, NH2-C(O)-CH2-C(O)-NH2 were determined by vibrational spectroscopy and theoretical calculations (HF/6-31G*, B3PW91/6-31G*). Solid state Fourier transform infrared and Raman spectra were analysed. They reveal the existence of a diketo tautomer. Theoretical calculations predict a
Alok Rout et al.
Dalton transactions (Cambridge, England : 2003), 43(4), 1862-1872 (2013-11-22)
A new non-fluorinated malonamide-based ionic liquid extractant was synthesized and investigated for the extraction behavior of europium(III) and other trivalent rare-earth ions from nitric acid medium. The extractant was the functionalized ionic liquid trihexyl(tetradecyl)phosphonium N,N,N',N'-tetra(2-ethylhexyl)malonate, [P66614][MA], and it was used
Syntheses and antiinflammatory activity of malonamic acid, malonamate and malonamide derivatives of some heterocyclic compounds.
T Katagi et al.
Chemical & pharmaceutical bulletin, 33(11), 4878-4888 (1985-11-01)
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico