1A00070
USP
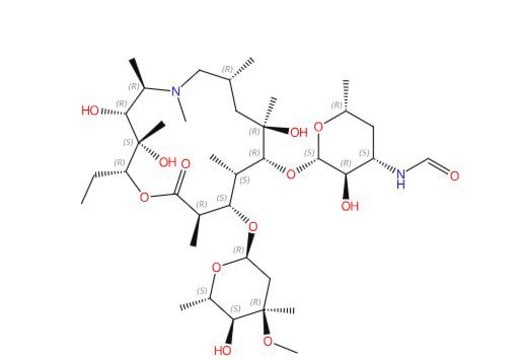
3′-(N,N-Didemethyl)Azithromycin; (Aminoazithromycin)
Pharmaceutical Analytical Impurity (PAI)
别名:
((2R,3S,4R,5R,8R,10R,11R,12S,13S,14R)-11-(((2S,3R,4S,6R)-4-amino-3-hydroxy-6-methyltetrahydro-2H-pyran-2-yl)oxy)-2- ethyl-3,4,10-trihydroxy-13-(((2R,4R,5S,6S)-5-hydroxy-4-methoxy-4,6-dimethyltetrahydro-2H-pyran-2-yl)oxy)- 3,5,6,8,10,12,14-heptamethyl-1-oxa-6-azacyclopentadecan-15-one, (2R,3S,4R,5R,8R,10R,11R,12S,13S,14R)-13-[(2,6-Dideoxy-3-C-methyl-3-O-methyl-α-L-ribo-hexopyranosyl)oxy]-2-ethyl3,4,10-trihydroxy-3,5,6,8,10,12,14-heptamethyl-11-[[3-amino-3,4,6-trideoxy-β-D-xylo-hexopyranosyl]oxy]-1-oxa-6- azacyclopentadecan-15-one)
登录查看公司和协议定价
所有图片(1)
About This Item
推荐产品
等級
pharmaceutical analytical impurity (PAI)
agency
USP
API 家族
azithromycin
製造商/商標名
USP
應用
pharmaceutical
格式
neat
儲存溫度
2-8°C
一般說明
3′-(N,N-Didemethyl)Azithromycin; (Aminoazithromycin) is a USP Pharmaceutical Analytical Impurity (PAI).
USP PAI are a product line of impurities suitable for research and analytical purposes, which help to ensure the quality and safety of medicines.
Associated Drug Substance: Azithromycin
Therapeutic Area: Antibiotics.
For more information about this PAI, visit here.
USP PAI are a product line of impurities suitable for research and analytical purposes, which help to ensure the quality and safety of medicines.
Associated Drug Substance: Azithromycin
Therapeutic Area: Antibiotics.
For more information about this PAI, visit here.
應用
3′-(N,N-Didemethyl)Azithromycin; (Aminoazithromycin) (USP PAI) is intended for use in analytical testing to detect, identify, and measure pharmaceutical impurities.
特點和優勢
USP PAI advance your early analytical R&D and process development. PAI can be used in the following applications:
1. Conduct analytical tests during early formulation feasibility studies.
2. Determine degradation impurities produced during stress studies.
3. Develop, validate, and transfer analytical methods.
4. Perform spiking studies during process R&D to demonstrate depletion upon recrystallization.
5. Record retention times and/or spectra
6. Determine relative response factors.
7. Identify unknown impurities that formed during ICH stability conditions.
8. Identify impurities that are present in the Reference Listed Drug
9. Test for and profile impurities not listed in drug substance and drug product monographs.
1. Conduct analytical tests during early formulation feasibility studies.
2. Determine degradation impurities produced during stress studies.
3. Develop, validate, and transfer analytical methods.
4. Perform spiking studies during process R&D to demonstrate depletion upon recrystallization.
5. Record retention times and/or spectra
6. Determine relative response factors.
7. Identify unknown impurities that formed during ICH stability conditions.
8. Identify impurities that are present in the Reference Listed Drug
9. Test for and profile impurities not listed in drug substance and drug product monographs.
分析報告
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
其他說明
Sales restrictions may apply.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门