SMB00928
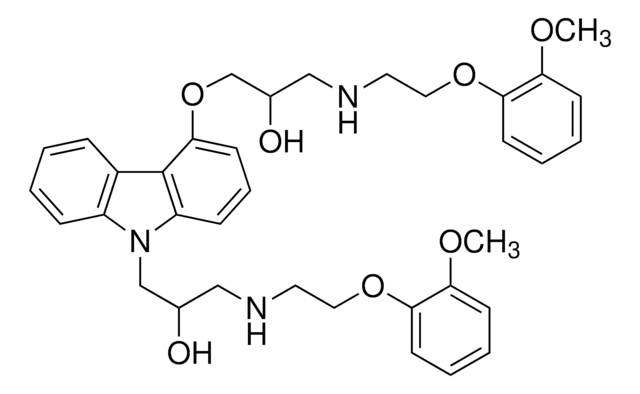
4′-Hydroxy carvedilol
别名:
4′-Hydroxy carvedilol, 4′-Hydroxyphenyl carvedilol, 4′-OH carvedilol, 4′OH-CVD, 4-(2-(3-(9H-carbazol-4-yloxy)-2-hydroxypropylamino)ethoxy)-3-methoxyphenol, 4-[2-[[3-(9H-Carbazol-4-yloxy)-2-hydroxypropyl]amino]ethoxy]-3-methoxyphenol, 4OHC, BM 140686, BM 14686, Carvedilol metabolite M4
登录查看公司和协议定价
所有图片(1)
About This Item
推荐产品
方案
≥95%
质量水平
表单
solid
颜色
off-white to white
储存温度
2-8°C
SMILES字符串
[nH]1c2c(c4c1cccc4)c(ccc2)OCC(O)CNCCOc3c(cc(cc3)O)OC
InChI
1S/C24H26N2O5/c1-29-23-13-16(27)9-10-21(23)30-12-11-25-14-17(28)15-31-22-8-4-7-20-24(22)18-5-2-3-6-19(18)26-20/h2-10,13,17,25-28H,11-12,14-15H2,1H3
InChI key
ZCJHEORDHXCJNB-UHFFFAOYSA-N
一般描述
Carvedilol is a β- and α1-adrenoreceptor blocker for the treatment of hypertension and congestive heart failure (CHF). The drug is metabolized by CYP2D6 (to 4′-OH and 5′-OH), CYP2C9 (to O-desmethyl), CYP1A2 (to 8-OH). It is also oxidized to 1-OH carvedilol, but the enzyme involved is not yet clear. These metabolites are useful markers for studying and monitoring the activities of cytochrome metabolizing enzymes. M4 metabolite (4′-OH), but not M2 or M5, is most likely to contribute to total β-adrenoceptor blocking activity due to its higher potency.
应用
Metabolomics
其他说明
For additional information on our range of Biochemicals, please complete this form.
警示用语:
Warning
危险声明
危险分类
Aquatic Chronic 2 - STOT RE 2
靶器官
Liver,spleen,Uterus (including cervix),Adrenal gland
储存分类代码
11 - Combustible Solids
WGK
WGK 3
闪点(°F)
Not applicable
闪点(°C)
Not applicable
D Tenero et al.
Journal of clinical pharmacology, 40(8), 844-853 (2000-08-10)
Carvedilol is a relatively new drug with beta- and alpha 1-receptor blocking activity and antioxidant effects recently approved for the treatment of congestive heart failure (CHF). An ascending, multiple-dose study was completed in 20 male patients with stable New York
W H Schaefer et al.
Drug metabolism and disposition: the biological fate of chemicals, 26(10), 958-969 (1998-10-08)
The excretion and biotransformation of carvedilol [1-[carbazolyl-(4)-oxy]-3-[(2-methoxyphenoxyethyl)amino]-2-p ropanol], a new, multiple-action, neurohormonal antagonist that exhibits the combined pharmacological activities of beta-adrenoreceptor antagonism, vasodilation, and antioxidation, were investigated in dogs, rats, and mice. Carvedilol was absorbed well, and biliary secretion was
Eben Jung et al.
Journal of Korean medical science, 33(27), e182-e182 (2018-07-03)
Carvedilol is commonly used to treat hypertension as a β- and α1-adrenoreceptor blocker, but it is metabolized by CYP2D6, and CYP2D6*10 allele is dominant in Asian population. The objective of this study was to assess the influence of CYP2D6 polymorphisms
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持