推荐产品
重組細胞
expressed in E. coli
品質等級
化驗
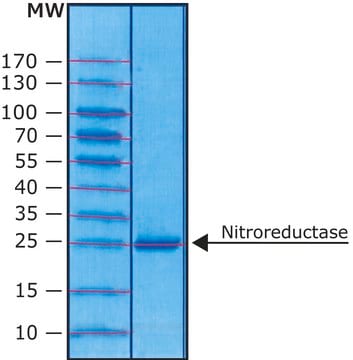
≥90% (SDS-PAGE)
形狀
lyophilized powder (one vial contains one mg protein)
比活性
≥100 units/mg protein
UniProt登錄號
儲存溫度
−20°C
基因資訊
human ... NQO2(4835)
應用
Quinone oxidoreductase 2 has been used in a study to investigate whether recent advances in limiting paraquat′s toxicity makes it safer for use as an herbicide. Quinone oxidoreductase 2 has also been used in a study to investigate the NQO2-mediated toxicity of the chemotherapeutic drug CB1954.
The product from Sigma has been used to identify novel inhibitors of the enzyme NQO2. It has also been used to investigate the effect of afobazole on quinone reductase 2 activity.
生化/生理作用
Modulation of NQO2 activity can have effects on the levels and functions of the tumour suppressor, p53. Deletion of NQO2 can alter intra-cellular signalling via the NF-κB pathway.
Unique human oxidoreductase that uses Dihydronicotinamide riboside (NRH) as a cosubstrate, not NAD(P)H. Activates anticancer prodrugs. Putative melatonin receptor.
單位定義
One unit will catalyze the oxidation of one micromole of 1-methyl-1,4-dihydronicotinamide to 1-methylnicotinamide per minute in the presence of menadione at pH 7.4 at 37 °C.
外觀
contains 10% PBS buffer salts
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
Effect of Afobazole on Activity of Quinone Reductase 2
Kadnikov IA, et al.
Pharmaceutical Chemistry Journal, 47(10), 514-516 (2014)
Karen A Nolan et al.
Molecular cancer therapeutics, 11(1), 194-203 (2011-11-18)
The National Cancer Institute chemical database has been screened using in silico docking to identify novel nanomolar inhibitors of NRH:quinone oxidoreductase 2 (NQO2). The inhibitors identified from the screen exhibit a diverse range of scaffolds and the structure of one
Paraquat Research. Do Recent Advances In Limiting Its Toxicity Make Its Use Safer?
Baltazar, T., et al.
British Journal of Pharmacology (2012)
K A Nolan et al.
Bioorganic & medicinal chemistry letters, 20(9), 2832-2836 (2010-04-02)
The purpose of the work was to identify novel inhibitors of the enzyme NQO2. Using computational molecular modelling, a QSAR (R(2)=0.88) was established, relating inhibitory potency with calculated binding affinity. From this, the imidazoacridin-6-one, NSC660841, was identified as the most
Faisal Hayat et al.
International journal of molecular sciences, 22(3) (2021-01-28)
As catabolites of nicotinamide possess physiological relevance, pyridones are often included in metabolomics measurements and associated with pathological outcomes in acute kidney injury (AKI). Pyridones are oxidation products of nicotinamide, its methylated form, and its ribosylated form. While they are
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门