推荐产品
生物源
Echis carinatus
化驗
≥90% (HPLC)
形狀
powder
技術
flow cytometry: suitable
inhibition assay: suitable
儲存溫度
−20°C
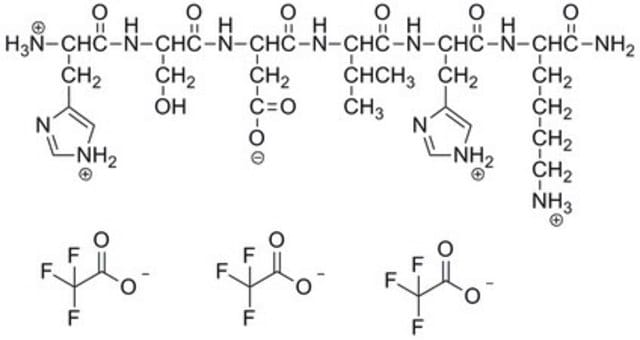
Amino Acid Sequence
Glu-Cys-Glu-Ser-Gly-Pro-Cys-Cys-Arg-Asn-Cys-Lys-Phe-Leu-Lys-Glu-Gly-Thr-Ile-Cys-Lys-Arg-Ala-Arg-Gly-Asp-Asp-Met-Asp-Asp-Tyr-Cys-Asn-Gly-Lys-Thr-Cys-Asp-Cys-Pro-Arg-Asn-Pro-His-Lys-Gly-Pro-Ala-Thr
一般說明
Echistatin is a single chain 49 amino acid residue protein, which prevents the aggregation of platelets. It has an isoelectric point (pI) of 8.3 and a molecular weight of 5400. This peptide is present in the venom of Echis carinatus, which is a saw-scaled viper. It contains the arginine-glycine-aspartic (RGD) acid sequence, which is present in proteins that bind to glycoprotein IIb/IIIa complex. It shares the proline-arginine-asparagine-proline sequence with the Aα chain of human fibrinogen. This protein is a member of disintegrin family, which prevents cell adhesion. With regards to molecular weight, echistatins are the smallest member of disintegrin family, and contains four isoforms called, α1, α2, β and γ.
應用
Echistatin has been used:
- as an inhibitor of integrin function to study the role of microfibril-associated glycoprotein-1 (Magp1) in the morphogenesis of vascular structures
- for the preparation of microbubbles targeted to αvβ3 integrins in tumor angiogenesis imaging
- as a conjugate to αvβ3 in flow cytometric binding studies
生化/生理作用
Disintegrins represent a novel family of integrin β1 and β3 inhibitor proteins isolated from viper venoms. They are low molecular-weight, cysteine-rich peptides containing the Arg-Gly-Asp (RGD) sequence. They are the most potent known inhibitors of integrin function. Disintegrins interfere with cell adhesion to the extracellular matrix, including adhesion of melanoma cells and fibroblasts to fibronectin, and are potent inhibitors of platelet aggregation.
Echistatin is a disintegrin, which prevents the aggregation of platelets. They interact with and prevent the binding of fibrinogen to their receptors on the membrane of platelets. It also inhibits platelet aggregation mediated by epinephrine, thrombin, collagen, or platelet-activating factor. Studies in isolated osteoclasts show that this peptide inhibits bone resorption by osteoclasts, most probably by damaging adhesion structures.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
M Gawaz et al.
Circulation, 96(6), 1809-1818 (1997-10-10)
Platelet interaction with endothelium plays an important role in the pathophysiology of coronary microcirculation. We assessed the role of the vitronectin receptor (integrin alpha(v)beta3) in platelet/endothelium adhesion. We investigated the effect on platelet/endothelium adhesion of plasma obtained from patients with
Z R Gan et al.
The Journal of biological chemistry, 263(36), 19827-19832 (1988-12-25)
A 49-residue protein, echistatin, which inhibits platelet aggregation, was purified from the venom of the saw-scaled viper Echis carinatus. The purification procedure included gel filtration on Sephadex G-50, cation-exchange chromatography on Mono S, and C18 reverse-phase high pressure liquid chromatography.
C C Kumar et al.
The Journal of pharmacology and experimental therapeutics, 283(2), 843-853 (1997-11-14)
Echistatin is a 49-amino-acid peptide belonging to the family of disintegrins that are derived from snake venoms and are potent inhibitors of platelet aggregation and cell adhesion. Integrin alphavbeta3 receptor plays a critical role in several physiological processes such as
Dilantha B Ellegala et al.
Circulation, 108(3), 336-341 (2003-07-02)
Angiogenesis is a critical determinant of tumor growth and metastasis. We hypothesized that contrast-enhanced ultrasound (CEU) with microbubbles targeted to alpha(v)-integrins expressed on the neovascular endothelium could be used to image angiogenesis. Malignant gliomas were produced in 14 athymic rats
M Sato et al.
The Journal of cell biology, 111(4), 1713-1723 (1990-10-01)
The venom protein, s-echistatin, originally derived from the saw-scaled viper Echis carinatus, was found to be a potent inhibitor of bone resorption by isolated osteoclasts. This Arg24-Gly25-Asp26-(RGD)-containing protein inhibited the excavation of bone slices by rat osteoclasts (IC50 = 0.1
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门