所有图片(1)
About This Item
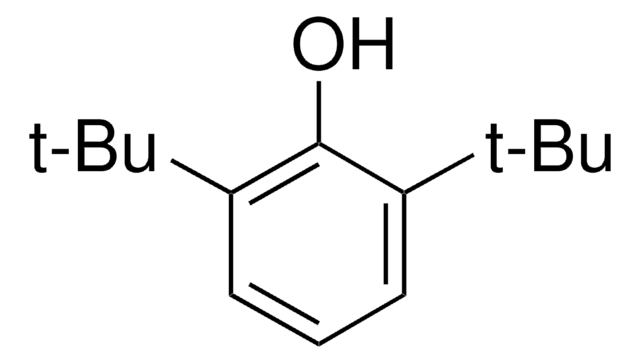
线性分子式:
[(CH3)3C]2C6H3OH
CAS号:
分子量:
206.32
Beilstein:
1841887
EC號碼:
MDL號碼:
分類程式碼代碼:
12000000
推荐产品
蒸汽壓力
<0.01 mmHg ( 20 °C)
化驗
~99% (GC)
bp
253 °C (lit.)
mp
34-37 °C (lit.)
儲存溫度
2-8°C
SMILES 字串
CC(C)(C)c1cccc(c1O)C(C)(C)C
InChI
1S/C14H22O/c1-13(2,3)10-8-7-9-11(12(10)15)14(4,5)6/h7-9,15H,1-6H3
InChI 密鑰
DKCPKDPYUFEZCP-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
取代透過
产品编号
说明
价格
訊號詞
Warning
危險聲明
危險分類
Aquatic Acute 1 - Aquatic Chronic 1 - Skin Irrit. 2
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 2
閃點(°F)
227.3 °F - closed cup
閃點(°C)
108.5 °C - closed cup
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
Mehmet Tümer et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 70(3), 477-481 (2007-09-07)
Crystals of the 3,3'-5,5'-tetra-tert-butyl-4,4'-diphenoquinone (TTBDQ) in the reaction mixture DCM/MeOH (1:1, v/v) were obtained as a result of CC coupling reaction of the sterically hindered phenol (2,6-di-tert-butylphenol, DTBP) using the binuclear Co(II) complexes. The oxidation product (TTBDQ), C(28)H(40)O(2), crystallizes in
G Haeseler et al.
European journal of anaesthesiology, 20(3), 220-224 (2003-03-26)
Propofol is a phenol derivative (2,6 di-isopropylphenol) with a unique effect profile including activating effects on GABA(A) and blocking effects on voltage-operated sodium channels. If the substituents in the 2- and the 6-positions are replaced by tert-butyl groups, the resulting
A W Girotti et al.
Lipids, 22(6), 401-408 (1987-06-01)
The effects of singlet oxygen- and oxygen radical-induced lipid peroxidation on cell membrane integrity were compared, using the human erythrocyte ghost as a model system. Resealed ghosts underwent lipid peroxidation and lysis (release of trapped glucose-6-P) when irradiated in the
Shinsuke Ishihara et al.
Journal of the American Chemical Society, 133(40), 16119-16126 (2011-08-31)
Porphyrin derivatives bearing 2,6-di-tert-butylphenol substituents at their 5,15-positions undergo reversible photoredox switching between porphyrin and porphodimethene states as revealed by UV-vis spectroscopy, fluorescence spectroscopy, and X-ray single-crystal analyses. Photoredox interconversion is accompanied by substantial variations in electronic absorption and fluorescence
Studies on the synthesis and anti-inflammatory activity of 2,6-di-tert-butylphenols with a heterocyclic group at the 4-position. V. Elimination reaction of the sulfinyl group of 2,3-dihydroimidazo[2,1-b]thiazole 1-oxide.
Y Isomura et al.
Chemical & pharmaceutical bulletin, 32(12), 4726-4730 (1984-12-01)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门





![1,4-二叠氮双环[2.2.2]辛烷 ReagentPlus®, ≥99%](/deepweb/assets/sigmaaldrich/product/structures/366/129/a6ff4175-974d-4fac-9038-b35e508ef252/640/a6ff4175-974d-4fac-9038-b35e508ef252.png)



