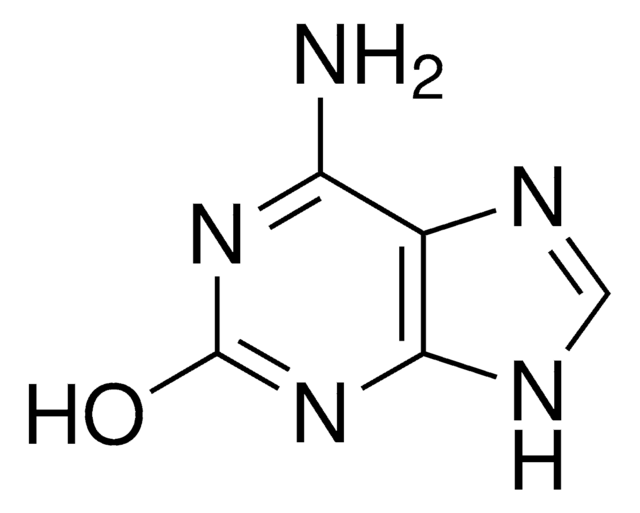
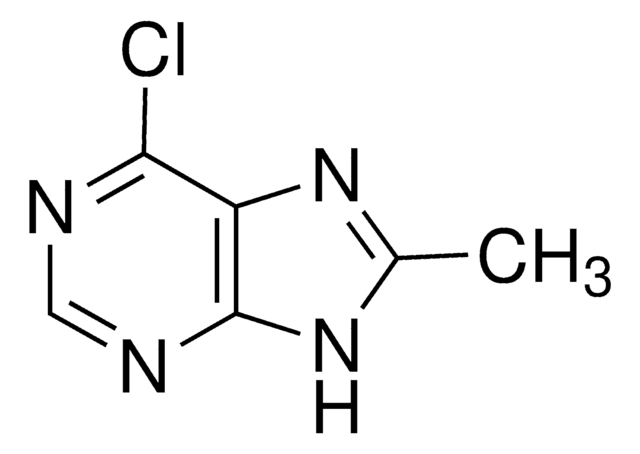
推荐产品
化驗
≥95%
形狀
powder
mp
165-167 °C (dec.) (lit.)
溶解度
water: 10 mg/mL, clear to very slightly hazy, colorless to faintly yellow
儲存溫度
−20°C
SMILES 字串
Nc1nc(Cl)c2ncn([C@@H]3O[C@H](CO)[C@@H](O)[C@H]3O)c2n1
InChI
1S/C10H12ClN5O4/c11-7-4-8(15-10(12)14-7)16(2-13-4)9-6(19)5(18)3(1-17)20-9/h2-3,5-6,9,17-19H,1H2,(H2,12,14,15)/t3-,5-,6-,9-/m1/s1
InChI 密鑰
TXWHPSZYRUHEGT-UUOKFMHZSA-N
正在寻找类似产品? 访问 产品对比指南
應用
2-Amino-6-chloropurine riboside is used in the biosynthesis of mutagenic and prodrug nucleosides such as 2′-deoxy-2-(p-nitrophenyl)-adenosine and 6-deoxyacyclovir and of 6-arylthio analogues.
儲存類別代碼
13 - Non Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
A Matsuda et al.
Nucleic acids symposium series, 17(17), 141-143 (1986-01-01)
The reaction of 2-amino-6-chloropurine riboside with i-amyl nitrite in benzene in the presence of Cu2O, followed by treatment with NH3/MeOH gave 2-phenyladenosine (1). The crude sample of 1 was found to be mutagenic to bacteria (Salmonella typhimurium TA 98 and
Takayoshi Torii et al.
Nucleosides, nucleotides & nucleic acids, 25(4-6), 655-665 (2006-07-15)
A key compound, 2-amino-6-chlor-9-(2,3-dideoxy-3-fluoro-beta-D-erythro-pentofuranosyl)puine, was prepared from 2-amino-6-chloropurine riboside in 5 steps, then subjected to the nucleophilic displacement with benzenethiols to afford 6-arylthio congeners. These compounds showed a similar anti-HBV effect to that of 2',3' dideoxy-3'-fluoroguanosine.
Alicja Stachelska-Wierzchowska et al.
Molecules (Basel, Switzerland), 24(8) (2019-04-19)
Etheno-derivatives of guanine, O6-methylguanine, and isoguanine were prepared and purified using standard methods. The title compounds were examined as potential substrates of purine-nucleoside phosphorylases from various sources in the reverse (synthetic) pathway. It was found that 1,N2-etheno-guanine and 1,N6-etheno-isoguanine are
J T Kusmierek et al.
Acta chemica Scandinavica. Series B: Organic chemistry and biochemistry, 41(10), 701-707 (1987-11-01)
D.c. polarography of 2-amino-6-chloropurine in aqueous medium over a broad pH range revealed two diffusion waves, the first of which corresponds to reduction of the C(6)-Cl bond, leading to formation of 2-aminopurine in high yield. Condensation of the sodium salt
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门