推荐产品
生物来源
synthetic
方案
≥97% (HPLC)
表单
solid
旋光性
[α]/D -26.5±2.0°, c = 1 in H2O
技术
HPLC: suitable
颜色
white to off-white
储存温度
2-8°C
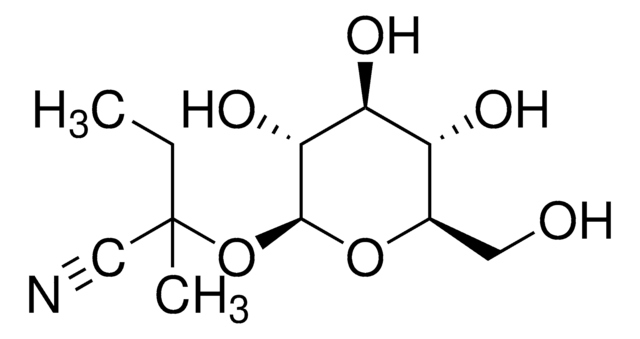
SMILES字符串
CC(C)(O[C@@H]1O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1O)C#N
InChI
1S/C10H17NO6/c1-10(2,4-11)17-9-8(15)7(14)6(13)5(3-12)16-9/h5-9,12-15H,3H2,1-2H3/t5-,6-,7+,8-,9+/m1/s1
InChI key
QLTCHMYAEJEXBT-ZEBDFXRSSA-N
正在寻找类似产品? 访问 产品对比指南
警示用语:
Warning
危险分类
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
靶器官
Respiratory system
储存分类代码
11 - Combustible Solids
WGK
WGK 3
闪点(°F)
Not applicable
闪点(°C)
Not applicable
个人防护装备
dust mask type N95 (US), Eyeshields, Gloves
Vega García-Escudero et al.
Autophagy, 4(7), 923-925 (2008-08-22)
The understanding of the mechanisms of cell-death execution and the role that they play in different diseases opens new therapeutic strategies. Currently, increasing evidence indicates that autophagy is a frequent cell-death mechanism, so the question arises: Could autophagy stimulation be
Karin Forslund et al.
Plant physiology, 135(1), 71-84 (2004-05-04)
Lotus japonicus was shown to contain the two nitrile glucosides rhodiocyanoside A and rhodiocyanoside D as well as the cyanogenic glucosides linamarin and lotaustralin. The content of cyanogenic and nitrile glucosides in L. japonicus depends on plant developmental stage and
Kirsten Jørgensen et al.
Plant physiology, 155(1), 282-292 (2010-11-04)
Cassava (Manihot esculenta) is a eudicotyledonous plant that produces the valine- and isoleucine-derived cyanogenic glucosides linamarin and lotaustralin with the corresponding oximes and cyanohydrins as key intermediates. CYP79 enzymes catalyzing amino acid-to-oxime conversion in cyanogenic glucoside biosynthesis are known from
Mika Zagrobelny et al.
Insect biochemistry and molecular biology, 37(11), 1189-1197 (2007-10-06)
Zygaena larvae sequester the cyanogenic glucosides (CNglcs) linamarin and lotaustralin from their food plants (Fabaceae) and also de novo biosynthesize these compounds. In Zygaenidae, CNglcs serve as defence compounds during the entire life cycle, and their content and ratio are
Hung Su et al.
Journal of food and drug analysis, 27(2), 415-427 (2019-04-17)
The unintentional ingestion of toxic compounds in herbs is not uncommon in many parts of the world. To provide timely and life-saving care in the emergency department, it is essential to develop a point-of-care analytical method that can rapidly identify
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持