推荐产品
化驗
96.0-102.0%
形狀
powder
抗生素活性譜
Gram-negative bacteria
Gram-positive bacteria
作用方式
cell wall synthesis | interferes
儲存溫度
2-8°C
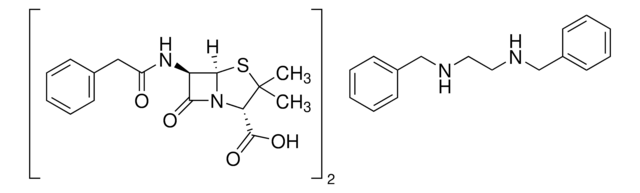
SMILES 字串
[Na+].[H][C@]12SC(C)(C)[C@@H](N1C(=O)[C@H]2NC(=O)Cc3ccccc3)C([O-])=O
InChI
1S/C16H18N2O4S.Na/c1-16(2)12(15(21)22)18-13(20)11(14(18)23-16)17-10(19)8-9-6-4-3-5-7-9;/h3-7,11-12,14H,8H2,1-2H3,(H,17,19)(H,21,22);/q;+1/p-1/t11-,12+,14-;/m1./s1
InChI 密鑰
FCPVYOBCFFNJFS-LQDWTQKMSA-M
正在寻找类似产品? 访问 产品对比指南
相关类别
一般說明
Chemical structure: ß-lactam
Penicillin G is a narrow spectrum natural antibiotic. It is effective against Streptococcus pneumoniae, groups A, B, C and G streptococci, nonenterococcal group D streptococci, viridans group streptococci, and non-penicillinase producing staphylococcus.
應用
Penicillin G has been used to study penicillin-binding protein 2, and non-toxigenic Corynebacterium diphtheriae isolated from cases of pharyngitis.
生化/生理作用
作用机制:青霉素G通过与青霉素结合蛋白(PBP)结合抑制肽聚糖链交联,从而抑制细胞壁合成。
抗菌谱:本品具有抗革兰氏阳性菌和革兰氏阴性菌活性。
抗菌谱:本品具有抗革兰氏阳性菌和革兰氏阴性菌活性。
包裝
1 G, 5 G
注意
Solutions should be filter sterilized and stored at 2-8°C for 1 week or at -20°C for more lengthy periods. Solutions are stable at 37°C for 3 days. The sodium salt is soluble in H2O at 100 mg/mL.
其他說明
Keep container tightly closed in a dry and well-ventilated place. Product contains Penicillin.
訊號詞
Warning
危險聲明
危險分類
Skin Sens. 1
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
個人防護裝備
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
其他客户在看
C von Hunolstein et al.
The Journal of antimicrobial chemotherapy, 50(1), 125-128 (2002-07-04)
Twenty-four strains of non-toxigenic Corynebacterium diphtheriae biotype gravis from the throats of patients with pharyngitis/tonsillitis were assayed for susceptibility to penicillin and erythromycin using determination of MIC, MBC and time-kill curves. There were no differences between the MICs of penicillin
Paul P Drury et al.
Neuropharmacology, 83, 62-70 (2014-04-15)
Basal ganglia injury after hypoxia-ischemia remains common in preterm infants, and is closely associated with later cerebral palsy. In the present study we tested the hypothesis that a highly selective neuronal nitric oxide synthase (nNOS) inhibitor, JI-10, would improve survival
Susumu Ochiai et al.
The Journal of antimicrobial chemotherapy, 60(1), 54-60 (2007-06-02)
In Neisseria gonorrhoeae, the mosaic structure of penicillin-binding protein 2 (PBP 2), composed of fragments of PBP 2 from Neisseria cinerea and Neisseria perflava, was significantly associated with decreased susceptibility to cephalosporins, particularly oral cephalosporins. The aim of this study
Muneki Hotomi et al.
PloS one, 8(3), e58124-e58124 (2013-03-14)
The protection against pneumococcal infections provided by currently available pneumococcal polysaccharide conjugate vaccines are restricted to the limited number of the serotypes included in the vaccine. In the present study, we evaluated the distribution of the pneumococcal capsular type and
Shuang Zhou et al.
Biosensors & bioelectronics, 49, 99-104 (2013-06-01)
Regulatory restrictions on antibiotic residues in dairy products have resulted in the illegal addition of β-lactamase to lower antibiotic levels in milk in China. Here we demonstrate a fast, sensitive and convenient method based on enzyme thermistor (ET) for the
商品
β-lactam antibacterials inhibit transpeptidase enzymes, preventing peptidoglycan assembly in both Gram-positive and Gram-negative bacteria.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门