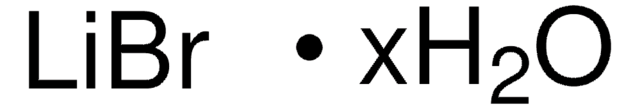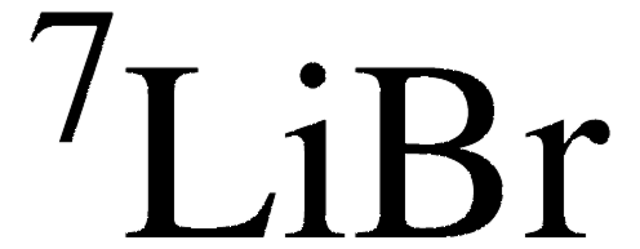推荐产品
蒸汽壓力
1 hPa ( 748 °C)
產品線
ReagentPlus®
化驗
≥99%
形狀
powder
pH值
7 (20 °C, 10 g/L)
mp
550 °C (lit.)
SMILES 字串
[Li+].[Br-]
InChI
1S/BrH.Li/h1H;/q;+1/p-1
InChI 密鑰
AMXOYNBUYSYVKV-UHFFFAOYSA-M
正在寻找类似产品? 访问 产品对比指南
一般說明
LiBr/三甲基氯硅烷试剂参与醇向溴化物的转换。
應用
溴化锂(LiBr)可用作以下研究的催化剂:
- 通过无溶剂二硫代缩醛反应将(芳族-和α,β-不饱和)醛转化为二硫代缩醛。
- 通过羰基化合物与活性亚甲基化合物的缩合反应合成烯烃。
- β-氨基醇的绿色合成。
- 通过使用LiBr/硝酸铈铵(CAN)试剂系统(作为Br+ 离子来源)进行芳族化合物的化学和区域选择性溴化。
法律資訊
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Redi-Dri is a trademark of Sigma-Aldrich Co. LLC
訊號詞
Warning
危險分類
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - Skin Sens. 1
儲存類別代碼
13 - Non Combustible Solids
水污染物質分類(WGK)
WGK 1
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
An efficient chemo and regioselective oxidative nuclear bromination of activated aromatic compounds using lithium bromide and ceric ammonium nitrate.
Roy SC, et al.
Tetrahedron Letters, 42(39), 6941-6942 (2001)
Lithium Bromide, an Inexpensive and Efficient Catalyst for Opening of Epoxide Rings by Amines at Room Temperature under Solvent-Free Condition.
Chakraborti AK, et al.
European Journal of Organic Chemistry, 2004(17), 3597-3600 (2004)
Lithium bromide as a new catalyst for carbon-carbon bond formation in the solid state.
Prajapati D, et al.
Journal of the Chemical Society. Perkin Transactions 1, 9, 959-960 (1996)
Lithium bromide-catalyzed highly chemoselective and efficient dithioacetalization of α,β-unsaturated and aromatic aldehydes under solvent-free conditions.
Firouzabadi H, et al.
Synthesis, 58-60 (1999)
Chlorotrimethylsilane/lithium bromide and hexamethyldisilane/pyridinium bromide perbromide: effective and selective reagents for the conversion of alkyl (cycloalkyl and aralkyl) alcohols into bromides.
Olah GA, et al.
The Journal of Organic Chemistry, 45(9), 1638-1639 (1980)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门