推荐产品
等級
pharmaceutical primary standard
API 家族
desogestrel
製造商/商標名
EDQM
應用
pharmaceutical (small molecule)
格式
neat
儲存溫度
2-8°C
SMILES 字串
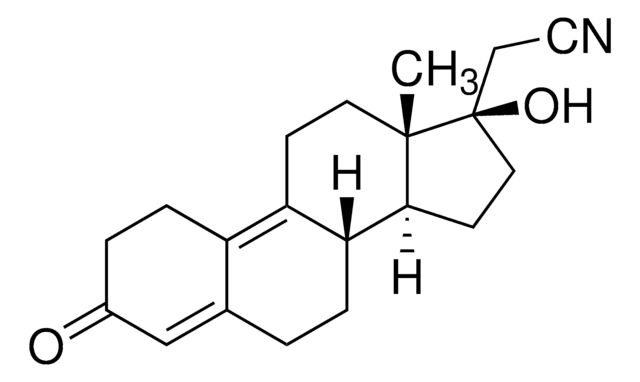
CC[C@]12CC(=C)[C@H]3[C@@H](CCC4=CCCC[C@H]34)[C@@H]1CC[C@@]2(O)C#C
InChI
1S/C22H30O/c1-4-21-14-15(3)20-17-9-7-6-8-16(17)10-11-18(20)19(21)12-13-22(21,23)5-2/h2,8,17-20,23H,3-4,6-7,9-14H2,1H3/t17-,18-,19-,20+,21-,22-/m0/s1
InChI 密鑰
RPLCPCMSCLEKRS-BPIQYHPVSA-N
基因資訊
human ... PGR(5241)
正在寻找类似产品? 访问 产品对比指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Desogestrel for system suitability EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
相關產品
产品编号
说明
价格
訊號詞
Danger
危險聲明
危險分類
Aquatic Acute 1 - Aquatic Chronic 1 - Repr. 1B
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
Giuseppe Benagiano et al.
Annals of the New York Academy of Sciences, 997, 163-173 (2003-12-03)
Progestin-only minipills have been available for over three decades, yet their use has been limited, because of a documented lower efficacy when compared to pills that combine estrogen and progestin. The availability of a new low-dose progestin-only minipill containing 75
R T Burkman
American journal of obstetrics and gynecology, 168(3 Pt 2), 1033-1040 (1993-03-01)
Desogestrel is a gonane progestogen that in early studies had an improved ratio between desired progestational effects and undesired androgenic effects. A review of more than 50 clinical studies suggests that desogestrel differs from progestins currently used in oral contraception
P G Stubblefield
American journal of obstetrics and gynecology, 168(3 Pt 2), 1047-1052 (1993-03-01)
Epidemiologic research has shown that current low-dose estrogen oral contraceptives are associated with a low risk of vascular events (e.g., myocardial infarction, stroke, and venous thrombosis or thromboembolism). Yet questions still persist about the effects of low-dose oral contraceptives on
L Laurendeau
Canadian family physician Medecin de famille canadien, 42, 62-71 (1996-01-01)
A review of clinical trials of changes in lipoprotein composition in women receiving oral contraceptives containing desogestrel; a comparison of the trials' findings and a discussion of their clinical significance. Using MEDLINE, we searched for articles published in English and
D F Archer
American journal of obstetrics and gynecology, 170(5 Pt 2), 1550-1555 (1994-05-01)
Desogestrel is a highly selective gonane progestin. A monophasic formulation containing 150 micrograms of desogestrel and 30 micrograms of ethinyl estradiol has recently been approved as an oral contraceptive (OC) in the United States. Although desogestrel-containing formulations are new to
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门