推荐产品
等級
analytical standard
儲存期限
limited shelf life, expiry date on the label
技術
HPLC: suitable
gas chromatography (GC): suitable
bp
242 °C (lit.)
mp
98-100 °C (lit.)
應用
environmental
格式
neat
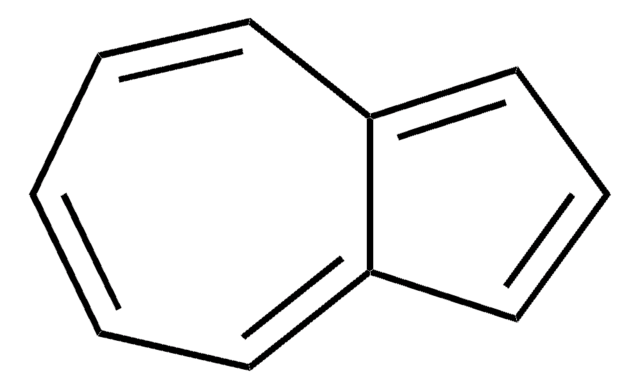
SMILES 字串
c1ccc2cccc2cc1
InChI
1S/C10H8/c1-2-5-9-7-4-8-10(9)6-3-1/h1-8H
InChI 密鑰
CUFNKYGDVFVPHO-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
Refer to the product′s Certificate of Analysis for more information on a suitable instrument technique. Contact Technical Service for further support.
推薦產品
Find a digital Reference Material for this product available on our online platform ChemisTwin® for NMR. You can use this digital equivalent on ChemisTwin® for your sample identity confirmation and compound quantification (with digital external standard). An NMR spectrum of this substance can be viewed and an online comparison against your sample can be performed with a few mouseclicks. Learn more here and start your free trial.
危險聲明
危險分類
Aquatic Chronic 2
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 2
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
Mine Ince et al.
Chemical communications (Cambridge, England), 48(34), 4058-4060 (2012-03-22)
A novel supramolecular electron donor-acceptor hybrid (2·1) and an electron donor-acceptor conjugate (3), both exhibiting a remarkably shifted Q band in the NIR region of the solar spectrum, were prepared. Irradiation of the supramolecular ensemble 2·1 within the visible range
Dawei Zhao et al.
The Journal of chemical physics, 131(18), 184307-184307 (2009-11-18)
The infrared (IR) spectrum of protonated azulene (AzuH(+), C(10)H(9)(+)) has been measured in the fingerprint range (600-1800 cm(-1)) by means of IR multiple photon dissociation (IRMPD) spectroscopy in a Fourier transform ion cyclotron resonance mass spectrometer equipped with an electrospray
Stefan Löber et al.
ChemMedChem, 4(3), 325-328 (2009-02-03)
Blue makes it happen: The non-uniform charge distribution of the blue colored azulene framework is highly suitable for the bioisosteric replacement of bicyclic heteroarene moieties. Showing an analogous binding mode as heterocyclic dopamine D4 receptor-selective lead compounds, the induction of
Atsuya Muranaka et al.
Journal of the American Chemical Society, 132(23), 7844-7845 (2010-05-27)
Azulenocyanine, having four azulene units fused to a tetraazaporphyrin skeleton, is a structural isomer of naphthalocyanine. We synthesized the first example of an azulenocyanine from 1,3-di-tert-butyl-5,6-dicyanoazulene. The macrocycle exhibits broad absorption over the visible and near-IR regions far beyond 1000
Timothy D Lash et al.
The Journal of organic chemistry, 77(5), 2368-2381 (2012-02-01)
A "2 + 2" strategy for synthesizing adj-dicarbaporphyrinoid systems has been developed. In a model study, an azulenylmethylpyrrole dialdehyde was condensed with a dipyrrylmethane in the presence of HCl, followed by oxidation with ferric chloride, to give a modest yield
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门