推荐产品
等級
analytical standard
品質等級
化驗
≥98.0% (HPLC)
儲存期限
limited shelf life, expiry date on the label
應用
food and beverages
格式
neat
儲存溫度
2-8°C
SMILES 字串
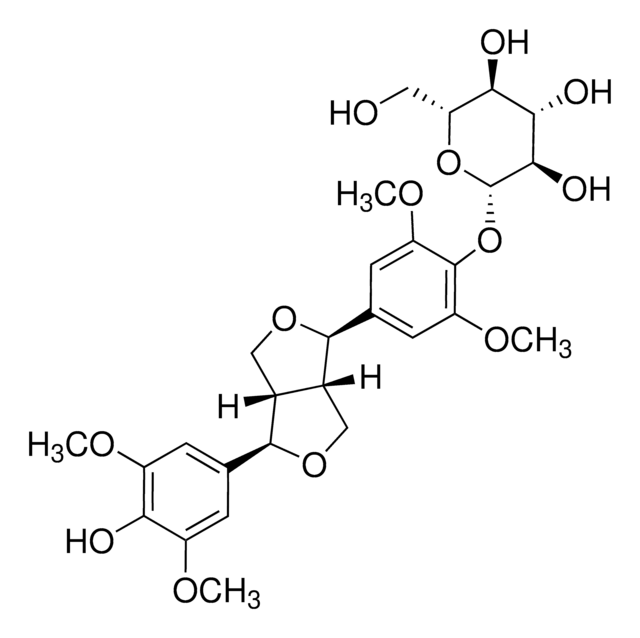
COc1cc(cc(OC)c1O[C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)[C@H]3OC[C@@H]4[C@@H]3CO[C@H]4c5cc(OC)c(O[C@@H]6O[C@H](CO)[C@@H](O)[C@H](O)[C@H]6O)c(OC)c5
InChI
1S/C34H46O18/c1-43-17-5-13(6-18(44-2)31(17)51-33-27(41)25(39)23(37)21(9-35)49-33)29-15-11-48-30(16(15)12-47-29)14-7-19(45-3)32(20(8-14)46-4)52-34-28(42)26(40)24(38)22(10-36)50-34/h5-8,15-16,21-30,33-42H,9-12H2,1-4H3/t15?,16?,21-,22+,23-,24+,25+,26-,27-,28+,29?,30?,33+,34-
InChI 密鑰
FFDULTAFAQRACT-RGFZIUCCSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
Eleutheroside E is one of the major bioactive saponins of Eleutherococcus senticosus.
應用
Eleutheroside E (ELU E) may be used as a reference standard for the analysis of ELU E in:
- Rat plasma and tissue by solid-phase extraction (SPE) followed by high-performance liquid chromatography (HPLC) and photodiode array detection (PDA).
- Acanthopanax senticosus by ionic liquids-ultrasound assisted extraction (ILUAE) followed by HPLC with ultraviolet (UV) detection.
- Eleutherococcus senticosus Maxim. by rapid resolution liquid chromatography (RRLC) equipped with multi-wavelength UV detector.
- Acanthopanax giraldii Harms by HPLC with diode array detector (DAD).
包裝
Bottomless glass bottle. Contents are inside inserted fused cone.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
D H Kim et al.
Archives of pharmacal research, 22(1), 30-34 (1999-03-11)
When liriodendrin or syringin was incubated for 24 h with human intestinal bacteria, two metabolites, (+)-syringaresinol-beta-D-glucopyranoside and (+)-syringaresinol, from liriodendrin and one metabolite, synapyl alcohol, from syringin were produced. The metabolic time course of liriodendrin was as follows: at early
Tomoko Sano et al.
Journal of natural medicines, 64(3), 257-265 (2010-03-11)
Boi and its original plant Sinomenium acutum from Japan were compared with Seifuto and its botanical origins from China in terms of their internal transcribed spacer (ITS) sequences and major chemical components. Boi, Seifuto, and their botanical origins overall showed
L B Kardono et al.
Journal of natural products, 53(6), 1447-1455 (1990-11-01)
By bioactivity-directed fractionation, six cytotoxic constituents have been characterized from the bark of Plumeria rubra collected in Indonesia. Three iridoids, fulvoplumierin [1], allamcin [2], and allamandin [3], as well as 2,5-dimethoxy-p-benzoquinone [4], were found to be active constituents of the
Yan-ru Deng et al.
Zhongguo Zhong yao za zhi = Zhongguo zhongyao zazhi = China journal of Chinese materia medica, 30(4), 272-274 (2005-02-24)
To study the chemical constituents from Lamium maculatum var. kansuense. The chemical constituents were isolated and repeatedly purified on silica gel column and the structures were elucidated by the NMR spectra and physico-chemical properties. Six compounds were obtained and identified
Chun Feng et al.
Archives of pharmacal research, 33(12), 1927-1932 (2010-12-31)
A new triterpene, 21-O-senecioyl-R(1)-barrigenol (1) and 13 known compounds were isolated from the ethanol extracts of the leaves and bark of Pittosporum brevicalyx (Oliv.) Gagnep. Their structures were elucidated based on spectral data. The antiarrhythmic action of one furofuran lignan
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门