推荐产品
等級
SAJ first grade
蒸汽密度
4 (vs air)
化驗
≥98.0%
形狀
solid
expl. lim.
6.3 %
存貨情形
available only in Japan
折射率
n20/D 1.439 (lit.)
pH值
12.4 (25 °C, 100 g/L)
mp
42-45 °C (lit.)
密度
0.89 g/mL at 25 °C (lit.)
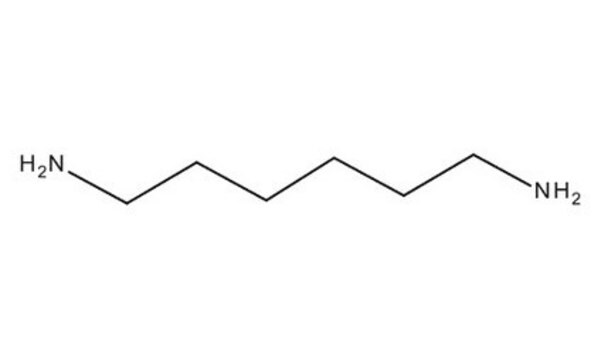
SMILES 字串
NCCCCCCN
InChI
1S/C6H16N2/c7-5-3-1-2-4-6-8/h1-8H2
InChI 密鑰
NAQMVNRVTILPCV-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
訊號詞
Danger
危險分類
Acute Tox. 4 Dermal - Acute Tox. 4 Oral - Eye Dam. 1 - Skin Corr. 1B - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
8A - Combustible corrosive hazardous materials
水污染物質分類(WGK)
WGK 1
閃點(°F)
176.0 °F - closed cup
閃點(°C)
80 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Linda G T Gaines et al.
Journal of environmental monitoring : JEM, 13(1), 119-127 (2010-10-28)
Although urinary 1,6-hexamethylene diamine (HDA) is a useful biomarker of exposure to 1,6-hexamethylene diisocyanate (HDI), a large degree of unexplained intra- and inter-individual variability exists between estimated HDI exposure and urine HDA levels. We investigated the effect of individual and
Richard F G Fröhlich et al.
Carbohydrate research, 346(12), 1592-1598 (2011-06-08)
Two simple and reliably accessible intermediates, N-carboxypentyl- and N-aminohexyl-1-deoxy-D-galactonojirimycin were employed for the synthesis of a set of terminally N-dansyl substituted derivatives. Reaction of the terminal carboxylic acid of N-carboxypentyl-1-deoxy-D-galactonojirimycin with N-dansyl-1,6-diaminohexane provided the chain-extended fluorescent derivative. Employing bis(6-dansylaminohexyl)amine, the
Junseok Yeom et al.
Bioconjugate chemistry, 21(2), 240-247 (2010-01-19)
A novel, biocompatible, and nontoxic dermal filler using hyaluronic acid (HA) hydrogels was successfully developed for tissue augmentation applications. Instead of using highly reactive cross-linkers such as divinyl sulfone (DVS) for Hylaform, 1,4-butanediol diglycidyl ether (BDDE) for Restylane, and 1,2,7,8-diepoxyoctane
Xiang Mei Yan et al.
Journal of biomaterials applications, 27(2), 179-186 (2011-04-30)
HA-HMDA hydrogels were developed by direct amide bond formation between the carboxyl groups of hyaluronic acid (HA) and hexamethylenediamine (HMDA) with an optimized carboxyl group modification in the preliminary experiment. However, these HA-HMDA hydrogels transformed into an unstable liquid form
Huina Zhang et al.
Biomaterials, 30(25), 4063-4069 (2009-06-03)
We previously established a simple method to immobilize the Arg-Gly-Asp (RGD) peptide on polycaprolactone (PCL) two-dimensional film surfaces that significantly improved bone marrow stromal cell (BMSC) adhesion to these films. The current work extends this modification strategy to three-dimensional (3D)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门