推荐产品
相关类别
一般說明
The protein named Proteinase-activated receptor 1 (PAR-1/PAR1) or Coagulation factor II receptor, or Thrombin receptor and encoded by the human gene F2R/CR2R/PAR1/TR is a high affinity receptor for activated thrombin and part of the innate immune system response driven by proteinase-activated receptors (PARs). PARs are G protein-coupled receptors that transmit cellular responses to extracellular proteases and have important functions in vascular physiology, development, inflammation, and cancer progression. PAR1 is important for platelet activation and vascular development. PAR1 expression is limited to platelets and vascular endothelial cells and is localized to the cell membrane. Interestingly too, even the signal peptide of PAR1 (which guides the protein to the membrane) plays a role in cell signaling as if forms the intracellular angiogenesis inhibitor peptide Parstatin.
免疫原
Linear peptide corresponding to human Par-1.
應用
Flow Cytometry Analysis: 4 µg from a representative lot detected Par-1 in 1X10E6 human thrombocytes (platelets).
Flow Cytometry Analysis: A representative lot from an independent laboratory detected Par-1 in CHRF-288 and HEL cells (Hoxie, J. A., et al. (1993). J Biol Chem. 268(18):13756-13763.).
Western Blotting Analysis: A representative lot from an independent laboratory detected Par-1 in human platelet membrane cell lysate (Brass, L. F., et al. (1992). J Biol Chem. 267(20):13795-13798.).
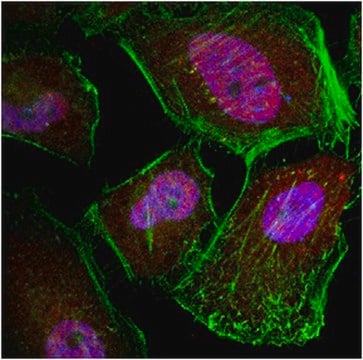
Immunocytochemistry Analysis: A representative lot from an independent laboratory detected Par-1 in CHRF-288 and HEL cells (Hoxie, J. A., et al. (1993). J Biol Chem. 268(18):13756-13763.).
Immunofluorescence Analysis: A representative lot from an independent laboratory detected Par-1 in CHRF-288 cells (Brass, L. F., et al. (1994). J Biol Chem. 269(4):2943-2952.).
Flow Cytometry Analysis: A representative lot from an independent laboratory detected Par-1 in CHRF-288 and HEL cells (Hoxie, J. A., et al. (1993). J Biol Chem. 268(18):13756-13763.).
Western Blotting Analysis: A representative lot from an independent laboratory detected Par-1 in human platelet membrane cell lysate (Brass, L. F., et al. (1992). J Biol Chem. 267(20):13795-13798.).
Immunocytochemistry Analysis: A representative lot from an independent laboratory detected Par-1 in CHRF-288 and HEL cells (Hoxie, J. A., et al. (1993). J Biol Chem. 268(18):13756-13763.).
Immunofluorescence Analysis: A representative lot from an independent laboratory detected Par-1 in CHRF-288 cells (Brass, L. F., et al. (1994). J Biol Chem. 269(4):2943-2952.).
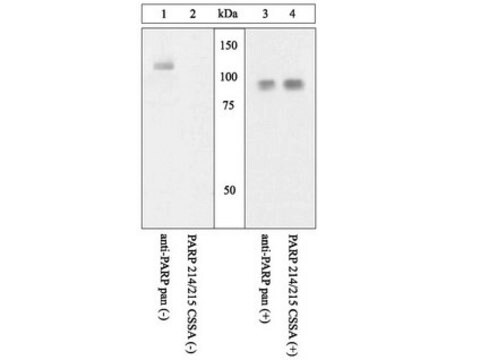
This Anti-PAR-1 antibody, clone ATAP2 is validated for use in western blotting, flow cytometry, ICC & immunofluorescence for the detection of PAR-1.
品質
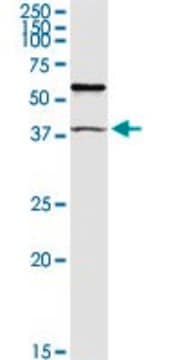
Evaluated by Western Blotting in mouse lymph node tissue.
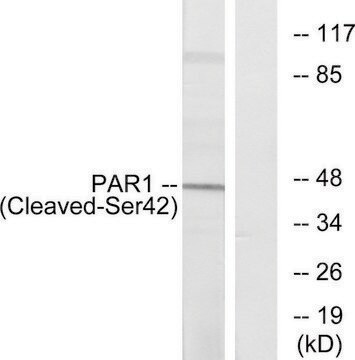
Western Blotting Analysis: 1 µg/mL of this antibody detected Par-1 in 10 µg of mouse lymph node tissue.
Western Blotting Analysis: 1 µg/mL of this antibody detected Par-1 in 10 µg of mouse lymph node tissue.
標靶描述
~50 kDa observed. Uncharacterized band(s) may be observed in some cell lysates.
外觀
Format: Purified
其他說明
Concentration: Please refer to the Certificate of Analysis for the lot-specific concentration.
Not finding the right product?
Try our 产品选型工具.
儲存類別代碼
12 - Non Combustible Liquids
水污染物質分類(WGK)
WGK 1
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Xiaofeng Cai et al.
Thrombosis and haemostasis, 121(11), 1448-1463 (2021-03-12)
Activated protein C (APC) is an anticoagulant plasma serine protease which exhibits potent cytoprotective and anti-inflammatory activities. Here, we studied protective effects of APC on the proinflammatory function of polyinosinic:polycytidylic acid [poly(I:C)], a synthetic analog of viral double-stranded RNA, in
Xiaofeng Cai et al.
Journal of thrombosis and haemostasis : JTH, 17(5), 803-817 (2019-03-14)
Essentials APC elicits cytoprotective responses in endothelial cells via EPCR-dependent cleavage of PAR1. APC inhibits LPS-mediated translocation and extracellular secretion of HMGB1 in endothelial cells. Signaling activity of APC inhibits LPS-mediated acetylation of HMGB1 by epigenetic mechanisms. APC inhibits LPS-mediated
Niraj Nag et al.
iScience, 27(6), 109828-109828 (2024-05-27)
We have purified Peptidase M84 from Bacillus altitudinis in an effort to isolate anticancer proteases from environmental microbial isolates. This metallo-protease had no discernible impact on normal cell survival, but it specifically induced apoptosis in ovarian cancer cells. PAR-1, a
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门