500586
JQ1 Enantiomers Set
别名:
JQ1 Enantiomers Set, (S)-tert-butyl 2-(4-(4-chlorophenyl)-2,3,9-trimethyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepin-6-yl)acetate, SGCBD01, BRD2 Inhibitor III, BRD3 Inhibitor II, BRD4 Inhibitor III, BRD6 Inhibitor I, BRDT Inhibitor I, BRD2 Inhibitor III, BRD3 Inhibitor II, BRD4 Inhibitor III, BRD6 Inhibitor I, BRDT Inhibitor I, (S)-tert-butyl 2-(4-(4-chlorophenyl)-2,3,9-trimethyl-6H-thieno[3,2-f][1,2,4]triazolo[4,3-a][1,4]diazepin-6-yl)acetate, SGCBD01
登录查看公司和协议定价
所有图片(1)
About This Item
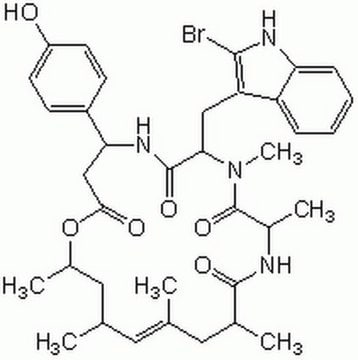
经验公式(希尔记法):
C23H25ClN4O2S
分子量:
456.99
分類程式碼代碼:
12352200
推荐产品
化驗
≥96% (for both enantiomers, HPLC)
品質等級
形狀
solid
效力
50 nM Ki
製造商/商標名
Calbiochem®
儲存條件
OK to freeze
protect from light
顏色
yellow-white
溶解度
DMSO: 50 mg/mL (Each compound is soluble)
運輸包裝
ambient
儲存溫度
−20°C
一般說明
A cell-permeable I-BET (Cat. No. 401010) class of thienodiazepine derivative whose (S)-(+), but not (R)-(-), enantiomer is shown to target both bromodomains (BD1 & BD2) of BET (bromodomain and extra terminal) family members BRD2 (KD = 128.4 nM for human BD1; ΔTmobs = 6.47 and 7.97 °C, respectively, for human BD1 and BD2), BRD3 (KD = 59.5 and 82.0 nM, respectively, for human BD1 and BD2), BRD4 (KD = 49.0 and 90.1 nM, respectively, for human BD1 and BD2), and BRD6/BRDT (KD = 190.1, 44.1, 77.5, and 59.2 nM, respectively, for human BD1, human BD2, murine BD1, and murine BD2) in a Kac- (ε-N-acetylated lysine) competitive manner, exhibiting little affinity toward 23 other BD-containing proteins, including the single BD-containing BRD1 and BRD9, and little or no activity against a panel of more than 50 receptors, ion channels, and transporters. Effectively inhibits the oncogenic BRD4-NUT (Nuclear protein in testis) fusion protein-dependent NMC (NUT midline carcinoma) and c-Myc oncoprotein-dependent MM (multiple myeloma) proliferation both in cultures (IC50<1 M) in vitro and in mice (50 mg/kg/day i.p.) in vivo. Also reported to cross the blood-testis boundary in male mice and effectively reduce testis mass, sperm count and motility (50 mg/kg; b.i.d. i.p.) in a reversible manner by targeting BRDT-mediated spermatogenesis without interfering hormone levels.
A cell-permeable I-BET (Cat. No. 401010) class of thienodiazepine derivative whose (S)-(+), but not (R)-(-), enantiomer is shown to target both bromodomains (BD1 & BD2) of BET family members BRD2, BRD3, BRD4, and BRD6/BRDT in a Kac- (ε-N-acetylated lysine) competitive manner, exhibiting little affinity toward 23 other BD-containing proteins, BRD1 and BRD9, and little or no activity against a panel of more than 50 receptors, ion channels, and transporters. Effectively inhibits the oncogenic BRD4-NUT fusion-dependent NUT midline carcinoma and c-Myc oncoprotein-dependent multiple myeloma proliferation both in cultures (IC50<1 M) in vitro and in mice (50 mg/kg/day i.p.) in vivo. Also reported to cross the blood-testis boundary in male mice and effectively block BRDT-mediated spermatogenesis without affecting hormone levels. The set contains 5 mg of the active (S)-(+)-JQ1 (540696) and 5 mg of the inactive (R)-(-) enantiomer (500585).
生化/生理作用
Cell permeable: yes
Primary Target
BRD4
BRD4
Reversible: yes
包裝
Packaged under inert gas
警告
Toxicity: Standard Handling (A)
外觀
Set contains 5 mg each of (S)-(+)-JQ1 enantiomer (540696-5MG) and (R)-(-) JQ1 enantiomer (500585-5MG)
重構
Following reconstitution, aliquot and freeze 9-20°C). Stock solutions are stable for up to 3 months at -20°C.
其他說明
Matzuk, M. M., et al. 2012. Cell.150, 673.
Delmore, J. E., et al. 2011 Cell.146, 904.
Filippakopoulos, P., et al. 2010. Nature.468, 1067.
Delmore, J. E., et al. 2011 Cell.146, 904.
Filippakopoulos, P., et al. 2010. Nature.468, 1067.
法律資訊
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
儲存類別代碼
11 - Combustible Solids
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门