444290
MMP Inhibitor V
The MMP Inhibitor V, also referenced under CAS 223472-31-9, controls the biological activity of MMP. This small molecule/inhibitor is primarily used for Protease Inhibitors applications.
别名:
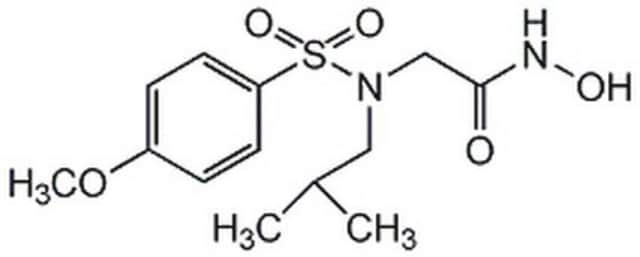
MMP Inhibitor V, (2S,4S)-N-Hydroxy-5-ethoxymethyloxy-2-methyl-4-(4-phenoxybenzoyl)aminopentanamide, ONO-4817
登录查看公司和协议定价
所有图片(1)
About This Item
推荐产品
品質等級
化驗
≥98% (HPLC)
形狀
solid
製造商/商標名
Calbiochem®
儲存條件
OK to freeze
protect from light
顏色
off-white
溶解度
ethanol: 15 mg/mL
DMSO: 30 mg/mL
運輸包裝
ambient
儲存溫度
2-8°C
InChI
1S/C22H28N2O6/c1-3-28-15-29-14-18(13-16(2)21(25)24-27)23-22(26)17-9-11-20(12-10-17)30-19-7-5-4-6-8-19/h4-12,16,18,27H,3,13-15H2,1-2H3,(H,23,26)(H,24,25)/t16-,18-/m0/s1
InChI 密鑰
HDWWQELUBWGQGA-WMZOPIPTSA-N
一般說明
An orally active non-peptidyl hydroxamate compound that acts as an effective broad-spectrum inhibitor against MMP-2, -3, -8, -9, -12, -13 (Ki = 0.73, 42, 1.1, 2.1, 0.45, and 1.1 nM, respectively), but not MMP-1 (IC50 = 1.6 µM), MMP-7 (Ki = 2.5 µM), or other serine proteases (no activity against chymotrypsin or plasmin at 100 µM). Widely used in studying MMP-mediated diseases development in vivo. Also reported to block P-LAP secretase activity (≥70% inhibition of P-LAP shedding at 10 µM) that is otherwise not inhibited by TIMP-1/2 in CHO cell cultures in vitro.
An orally active non-peptidyl hydroxamate compound that acts as an effective broad-spectrum inhibitor against MMP-2/3/8/9/12/13 (Ki = 0.73, 42, 1.1, 2.1, 0.45, and 1.1 nM, respectively), but not MMP-1 (IC50 = 1.6 µM), MMP-7 (Ki = 2.5 µM), or other serine proteases (no activity against chymotrypsin or plasmin at 100 µM). Widely used in studying MMP-mediated diseases development in vivo. Also reported to block P-LAP secretase activity (≥70% inhibition of P-LAP shedding at 10 µM) that is otherwise not inhibited by TIMP-1/2 in CHO cell cultures in vitro.
包裝
Packaged under inert gas
警告
Toxicity: Standard Handling (A)
其他說明
Okamoto, Y., et al. 2007. Int. Heart J.48, 369.
Ito, N., et al. 2004. Biochem. Biophys. Res. Commun.314, 1008.
Shiraga, M., et al. 2002. Cancer Res.62, 5967.
Mori, T., et al. 2001. Exp. Biol. Med.226, 429.
Yamada, A., et al. 2000. Inflamm. Res.49, 144.
Ito, N., et al. 2004. Biochem. Biophys. Res. Commun.314, 1008.
Shiraga, M., et al. 2002. Cancer Res.62, 5967.
Mori, T., et al. 2001. Exp. Biol. Med.226, 429.
Yamada, A., et al. 2000. Inflamm. Res.49, 144.
法律資訊
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 1
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Søren B van Witteloostuijn et al.
Journal of peptide science : an official publication of the European Peptide Society, 23(12), 845-854 (2017-10-24)
Bariatric surgery is currently the most effective treatment of obesity, which has spurred an interest in developing pharmaceutical mimetics. It is thought that the marked body weight-lowering effects of bariatric surgery involve stimulated secretion of appetite-regulating gut hormones, including glucagon-like
Prem Swaroop Yadav et al.
iScience, 26(9), 107548-107548 (2023-08-28)
Low circulating phosphate (Pi) leads to rickets, characterized by expansion of the hypertrophic chondrocytes (HCs) in the growth plate due to impaired HC apoptosis. Studies in HCs demonstrate that Pi activates the Raf/MEK/ERK1/2 and mitochondrial apoptotic pathways. To determine how
相关内容
Select different protease inhibitor types based on your needs to prevent protein degradation during isolation and characterization and safeguard proteins in sample prep.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门