推荐产品
化驗
≥99%
bp
220 °C (lit.)
mp
30-32 °C (lit.)
密度
1.049 g/mL at 25 °C (lit.)
儲存溫度
2-8°C
SMILES 字串
N#CCC#N
InChI
1S/C3H2N2/c4-2-1-3-5/h1H2
InChI 密鑰
CUONGYYJJVDODC-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
應用
- 碱促进的[1,6]-萘啶水上合成。†
- γ--酮酰胺的合成。
- 特殊杂环药物支架(包括吡啶、1,4-二氢吡啶、苯并吡喃[2,3-b]吡啶和二氢-1,4-二噻吩骨架)的制备。
包裝
訊號詞
Danger
危險分類
Acute Tox. 2 Oral - Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Irrit. 2 - Skin Sens. 1
儲存類別代碼
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
186.8 °F - closed cup
閃點(°C)
86 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
其他客户在看
商品
MOST offers controlled solar energy harvesting and storage, addressing global energy demands with improved storage techniques.
Knoevenagel Condensation is an organic reaction named after Emil Knoevenagel. It is a classic C-C bond formation reaction and a modification of the Aldol Condensation.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门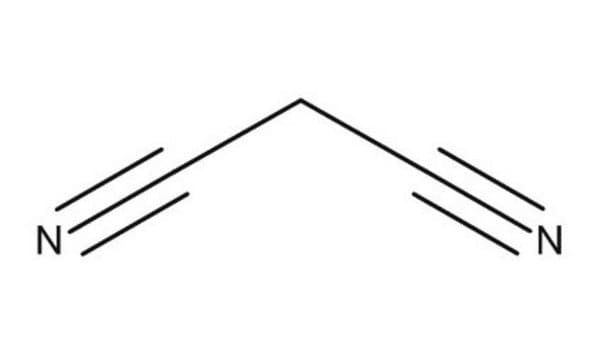












![2- [双(甲硫基)亚甲基]丙二腈 97%](/deepweb/assets/sigmaaldrich/product/structures/144/342/6a420594-3bce-4984-a8b7-5bf2a92d6a97/640/6a420594-3bce-4984-a8b7-5bf2a92d6a97.png)