推荐产品
品質等級
產品線
ReagentPlus®
化驗
99%
形狀
liquid
折射率
n20/D 1.592 (lit.)
bp
220-221 °C (lit.)
密度
1.063 g/mL at 25 °C (lit.)
SMILES 字串
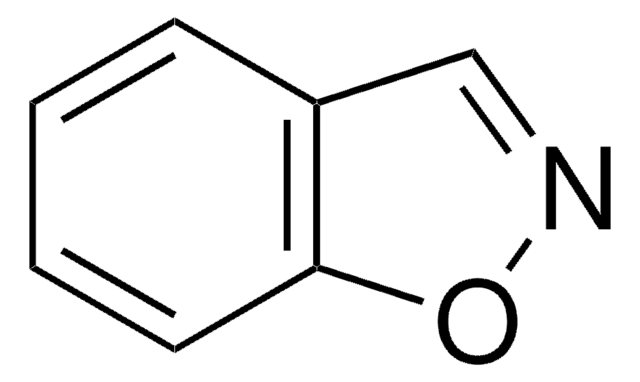
C1Cc2ccccc2N1
InChI
1S/C8H9N/c1-2-4-8-7(3-1)5-6-9-8/h1-4,9H,5-6H2
InChI 密鑰
LPAGFVYQRIESJQ-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
用作制备以下产品的反应物:
- NOD1 诱导的核因子-κB 活化抑制剂
- 1-磷酸鞘氨醇 4 (S1P4) 受体拮抗剂
- 细胞毒性细胞周期抑制剂
- 2-氨基吡啶类
- 用于蛋白激酶 C (PKC) 成像的 PET 试剂
- 治疗糖尿病高血糖症的钠依赖性葡萄糖协同转运蛋白 2 (SGLT2) 抑制剂
- α4β2-烟碱乙酰胆碱受体选择性部分激动剂
- mGlu4 阳性变构调节剂
- 细菌生物膜抑制剂
- 血清素 5-HT6 受体拮抗剂
法律資訊
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
199.4 °F - closed cup
閃點(°C)
93 °C - closed cup
個人防護裝備
Eyeshields, Gloves, multi-purpose combination respirator cartridge (US)
其他客户在看
Matej Baláž et al.
Molecules (Basel, Switzerland), 24(18) (2019-09-22)
Performing solution-phase oximation reactions with hydroxylamine hydrochloride (NH2OH·HCl) carries significant risk, especially in aqueous solutions. In the present study, four N-substituted indole-3-carboxaldehyde oximes were prepared from the corresponding aldehydes by solvent-free reaction with NH2OH·HCl and a base (NaOH or Na2CO3)
Toshiharu Noji et al.
Organic letters, 15(8), 1946-1949 (2013-04-04)
A benzyne-mediated synthesis of substituted indolines and carbazoles was developed. The reaction includes generation of benzyne using Mg(TMP)2·2LiCl as a base, cyclization, and trapping the resulting organomagnesium intermediate with an electrophile to provide a series of substituted indolines and carbazoles
Manas K Ghorai et al.
The Journal of organic chemistry, 78(8), 3867-3878 (2013-04-04)
A practical approach for the synthesis of 3-substituted indolines via regio- and stereoselective SN2-type ring-opening of 2-(2-halophenyl)-N-tosylaziridines with heteroatomic nucleophiles (O, N, and S) followed by palladium-catalyzed intramolecular C-N cyclization is reported in excellent yields (up to >99%) and enantiomeric
Tanguy Saget et al.
Organic letters, 15(6), 1354-1357 (2013-03-05)
The synthesis of cyclopropyl spiroindolines is described using an intramolecular palladium(0)-catalyzed C-H functionalization of a methine C(sp(3))-H bond. This transformation can be coupled with intermolecular Suzuki couplings or direct arylations of heteroaromatics to access functionalized indoline scaffolds in a single
A diversity-oriented approach to spiroindolines: post-Ugi gold-catalyzed diastereoselective domino cyclization.
Sachin G Modha et al.
Angewandte Chemie (International ed. in English), 51(38), 9572-9575 (2012-08-22)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门