推荐产品
品質等級
化驗
95%
形狀
liquid
折射率
n20/D 1.447 (lit.)
bp
95-98 °C/19 mmHg (lit.)
密度
1.156 g/mL at 25 °C (lit.)
SMILES 字串
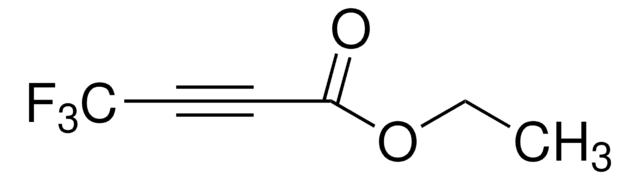
COC(=O)C#CC(=O)OC
InChI
1S/C6H6O4/c1-9-5(7)3-4-6(8)10-2/h1-2H3
InChI 密鑰
VHILMKFSCRWWIJ-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
乙炔二羧酸二甲酯 (DMAD)是一种酯,可用作环加成反应中的二烯亲和物和亲偶极物。
應用
用于 Diels-Alder 反应的多功能亲二烯体。
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Corr. 1B
儲存類別代碼
8A - Combustible corrosive hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
186.8 °F - closed cup
閃點(°C)
86 °C - closed cup
個人防護裝備
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
其他客户在看
Steven E Wheeler et al.
Journal of the American Chemical Society, 132(10), 3304-3311 (2010-02-18)
Stereoselective Diels-Alder cycloadditions that probe substituent effects in aryl-aryl sandwich complexes were studied experimentally and theoretically. Computations on model systems demonstrate that the stereoselectivity in these reactions is mediated by differential pi-stacking interactions in competing transition states. This allows relative
Yi Li et al.
Polymers, 13(3) (2021-01-28)
Big spherulite structure and high crystallinity are the two main drawbacks of poly(butylene succinate) (PBS) and hinder its application. In this work, a new type of copolyester poly(butylene succinate-co-butylene acetylenedicarboxylate) (PBSAD) is synthesized. With the incorporation of acetylenedicarboxylate (AD) units
Dimethyl acetylenedicarboxylate: A versatile tool in organic synthesis
CG Neochoritis, et al.
Synthesis, 537-585 (2014)
Qiuping Ding et al.
The Journal of organic chemistry, 74(2), 921-924 (2008-12-06)
Tandem electrophilic cyclization-[3+2] cycloaddition-rearrangement reactions of 2-alkynylbenzaldoximes, DMAD, and bromine are described, which afford the unexpected isoquinoline-based azomethine ylides in good to excellent yields. The products could be further elaborated via palladium-catalyzed cross-coupling reactions to generate highly functionalized isoquinoline-based stable
Cheng Ma et al.
The Journal of organic chemistry, 70(22), 8919-8923 (2005-10-22)
[reaction: see text] A facile preparation of 3-aminofuran derivatives via multicomponent reactions of thiazole carbenes, aldehydes, and dimethyl acetylenedicarboxylate (DMAD) is reported. In this process, the thiazole carbenes, generated in situ from thiazolium salts, reacted with aldehydes and DMAD at
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持