917672
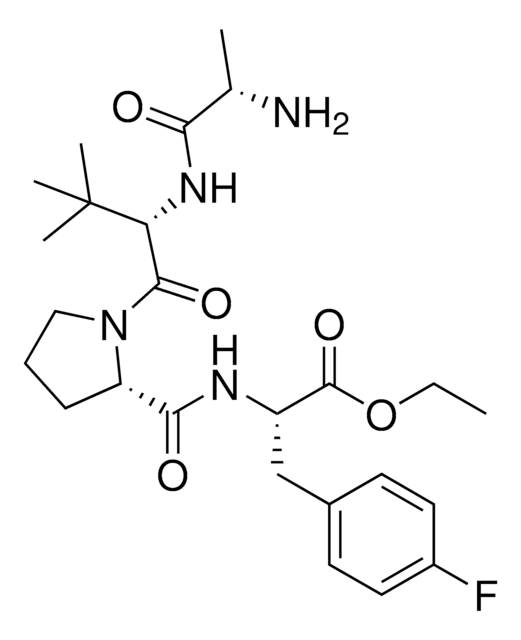
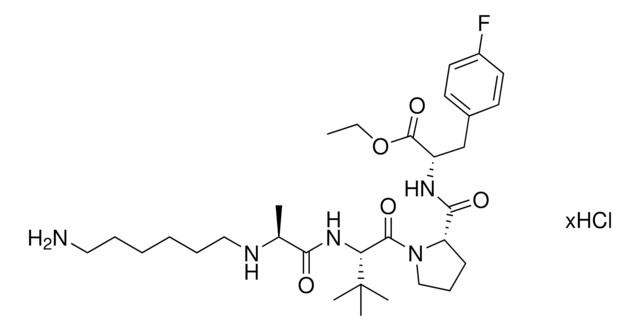
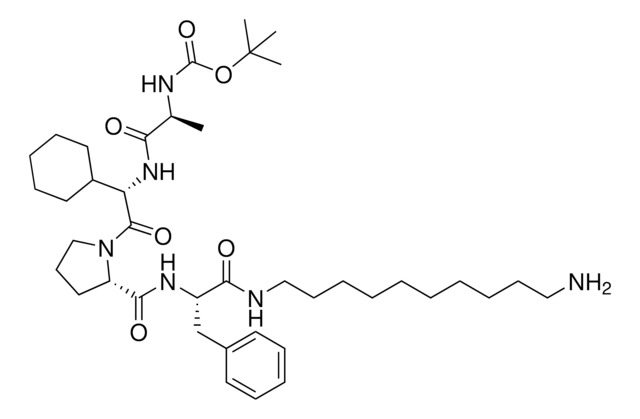
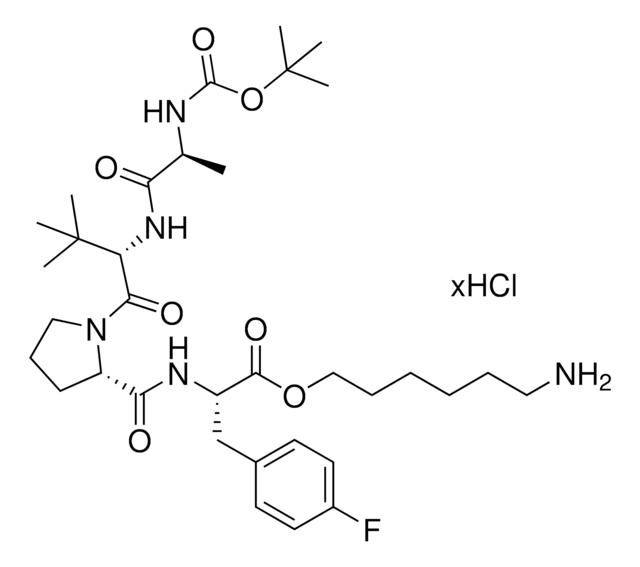
A1V1PF2-OEt-C10-NH2 hydrochloride
别名:
AVP conjugate for IAP-mediated protein degrader development, Ethyl (S)-2-((S)-1-((S)-2-((S)-2-((10-aminodecyl)amino)propanamido)-3,3-dimethylbutanoyl)pyrrolidine-2-carboxamido)-3-(4-fluorophenyl)propanoate, SNIPER building block
About This Item
推荐产品
ligand
A1V1PF2
品質等級
形狀
powder
反應適用性
reactivity: carboxyl reactive
reagent type: ligand-linker conjugate
官能基
amine
儲存溫度
2-8°C
SMILES 字串
C[C@H](NCCCCCCCCCCN)C(N[C@H](C(N1CCC[C@H]1C(N[C@H](C(OCC)=O)CC2=CC=C(C=C2)F)=O)=O)C(C)(C)C)=O.Cl
應用
Building blocks in this series:
917710 A1V1PF2-OEt
917427 A1V1PF2-OEt-C6-NH2 hydrochloride
917672A1V1PF2-OEt-C10-NH2 hydrochloride
917923 A1V1PF2-OEt-PEG1-NH2 hydrochloride
916676 A1V1PF2-OEt-PEG3-NH2 hydrochloride
Technology Spotlight: Degrader Building Blocks with Inhibitor of Apoptosis Protein (IAP) In Silico-Derived Ligands
其他說明
法律資訊
相關產品
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
商品
Plate of 80 ligands against E3 ligase IAP designed by ComInnex; allows creation of bifunctional targeted protein degraders or molecular glues.
Protein Degrader Building Blocks are a collection of crosslinker-E3 ligand conjugates with a pendant functional group for covalent linkage to a target ligand.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门