推荐产品
方案
≥95%
表单
powder
储存温度
−20°C
InChI
1S/C19H29NO4/c1-5-23-18(21)15-12(3)20-13(4)16(19(22)24-6-2)17(15)14-10-8-7-9-11-14/h14,17,20H,5-11H2,1-4H3
InChI key
GERWBKSVDHUVIT-UHFFFAOYSA-N
应用
Diethyl-4-cyclohexyl-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate was reported to chemoselectively modify histidine under visible light where the unsubstituted nitrogen groups on the modified His imidazole are conserved. Diethyl-4-cyclohexyl-2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate is also a versitile reagent for photoredox chemistry.
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
Product can be used with our line of photoreactors: Including Penn PhD (Z744035) & SynLED 2.0 (Z744080)
其他说明
Histidine-specific peptide modification via visible-light-promoted C-H alkylation
Open-Air Alkylation Reactions in Photoredox-Catalyzed DNA-Encoded Library Synthesis
A photocatalyst-free photo-induced denitroalkylation of ß-nitrostyrenes with 4-alkyl substituted Hantzsch esters at room temperature
Intermolecular Radical Addition to Ketoacids Enabled by Boron Activation
Oxa- and Azabenzonorbornadienes as Electrophilic Partners under Photoredox/Nickel Dual Catalysis
Exploration of a chiral cobalt catalyst for visible-light-induced enantioselective radical conjugate addition
Open-Air Alkylation Reactions in Photoredox-Catalyzed DNA-Encoded Library Synthesis
A photocatalyst-free photo-induced denitroalkylation of ß-nitrostyrenes with 4-alkyl substituted Hantzsch esters at room temperature
Intermolecular Radical Addition to Ketoacids Enabled by Boron Activation
Oxa- and Azabenzonorbornadienes as Electrophilic Partners under Photoredox/Nickel Dual Catalysis
Exploration of a chiral cobalt catalyst for visible-light-induced enantioselective radical conjugate addition
警示用语:
Warning
危险分类
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
靶器官
Respiratory system
储存分类代码
11 - Combustible Solids
WGK
WGK 3
闪点(°F)
Not applicable
闪点(°C)
Not applicable
Xuefeng Wang et al.
Chemical communications (Cambridge, England), 55(43), 6010-6013 (2019-05-08)
A sulfonylation reaction of 4-substituted Hantzsch esters, DABCO·(SO2)2, and electron-deficient alkenes at room temperature in the presence of photoredox catalysis under visible light irradiation is described. Not only (E)-chalcones but also (vinylsulfonyl)benzene and 2-vinylpyridine are all suitable substrates in the
Kaiqian Wang et al.
Organic & biomolecular chemistry, 17(15), 3845-3852 (2019-04-03)
Herein, we report a simple, economical, and effective acid-mediated method for the in situ deuteration of Hantzsch esters and their 4-substituted derivatives, including some drugs that constitute important calcium channel blockers which are effective for hypertension treatment. Hydrogen isotope exchange
Hai-Wu Du et al.
Organic letters, 22(4), 1542-1546 (2020-01-29)
In this study, a facile and efficient method to synthesize monofluoroalkenes by photoredox catalytic defluorinative alkylation of gem-difluoroalkenes with 4-alkyl-1,4-dihydropyridines under mild conditions (room temperature) is described. This novel strategy is applicable for a broad range of gem-difluoroalkene substrates with
Jennifer K Matsui et al.
Angewandte Chemie (International ed. in English), 57(48), 15847-15851 (2018-10-12)
A regioselective, nickel-catalyzed photoredox allylation of secondary, benzyl, and α-alkoxy radical precursors is disclosed. Through this manifold, a variety of linear allylic alcohols and allylated monosaccharides are accessible in high yields under mild reaction conditions. Quantum mechanical calculations [DFT and
Xuefeng Wang et al.
Chemical communications (Cambridge, England), 55(14), 2062-2065 (2019-01-29)
A three-component reaction between 4-substituted Hantzsch esters, DABCO·(SO2)2, and vinyl azides in the presence of photoredox catalysts under visible light irradiation is developed. Substituted Hantzsch esters as radical reservoirs are used in the reaction between sulfur dioxide and vinyl azides.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持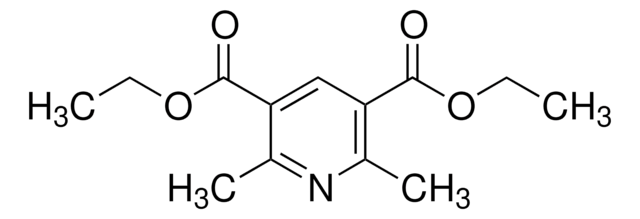






![[Ir(dtbbpy)(ppy)2]PF6](/deepweb/assets/sigmaaldrich/product/structures/158/329/2544d673-d267-4aa1-8f46-2652aad4bfa0/640/2544d673-d267-4aa1-8f46-2652aad4bfa0.png)

