推荐产品
化驗
≥97.5% (HPLC)
98%
形狀
powder
反應適用性
reaction type: Coupling Reactions
雜質
≤0.5% water
應用
peptide synthesis
SMILES 字串
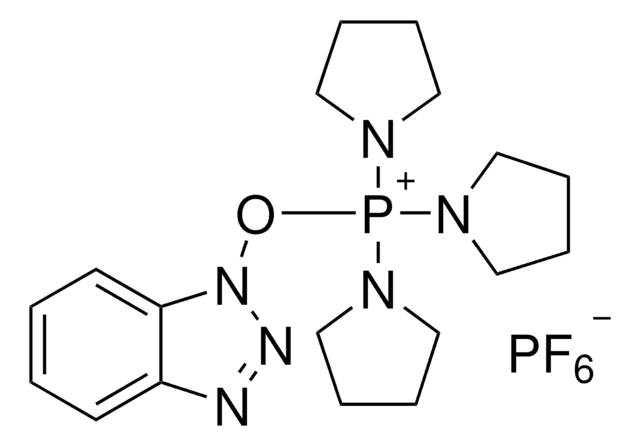
F[B-](F)(F)F.CN(C)C(\On1nnc2ccc(Cl)cc12)=[N+](/C)C
InChI
1S/C11H15ClN5O.BF4/c1-15(2)11(16(3)4)18-17-10-7-8(12)5-6-9(10)13-14-17;2-1(3,4)5/h5-7H,1-4H3;/q+1;-1
InChI 密鑰
GBGVQFJZGHBZMC-UHFFFAOYSA-N
應用
Coupling reagent for:
- Synthesis via plate-based multiple parallel centrifugation synthesizer
- Solid-phase synthesis of cyclic gramicidin analogs
- Comparison of automatic multiple peptide synthesizers
- Solid-phase peptide coupling with lack of racemization
訊號詞
Danger
危險聲明
危險分類
Flam. Sol. 1 - Skin Sens. 1A
安全危害
儲存類別代碼
4.1B - Flammable solid hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
其他客户在看
P Carvajal-Rondanelli et al.
Amino acids, 50(5), 557-568 (2018-02-23)
Previous work demonstrated that lysine homopeptides adopt a polyproline II (PPII) structure. Lysine homopeptides with odd number of residues, especially with 11 residues (K11), were capable of inhibiting the growth of a broader spectrum of bacteria than those with an
Martyna Kielmas et al.
Analytical and bioanalytical chemistry, 407(9), 2557-2567 (2015-02-01)
Glycation of α-crystallin is responsible for age- and diabetic-related cataracts, which are the main cause of blindness worldwide. We optimized the method of identification of lysine residues prone to glycation using the combination of LC-MS, isotopic labeling, and modified synthetic
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门