所有图片(1)
About This Item
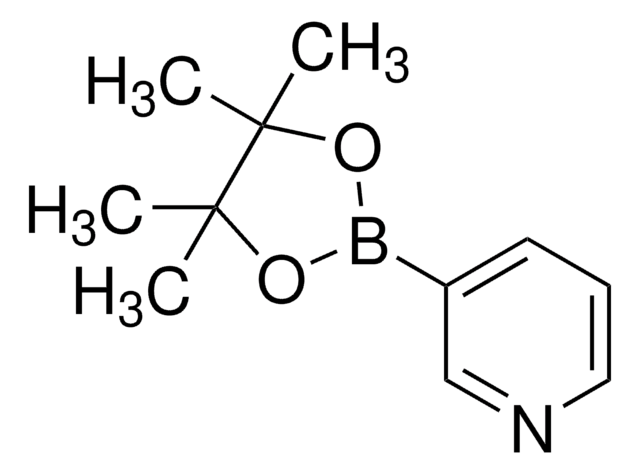
经验公式(希尔记法):
C10H14BNO2
CAS号:
分子量:
191.03
MDL號碼:
分類程式碼代碼:
12352103
PubChem物質ID:
NACRES:
NA.22
推荐产品
化驗
97%
形狀
solid
mp
92-96 °C (lit.)
SMILES 字串
CC1(C)COB(OC1)c2cccnc2
InChI
1S/C10H14BNO2/c1-10(2)7-13-11(14-8-10)9-4-3-5-12-6-9/h3-6H,7-8H2,1-2H3
InChI 密鑰
QMEKTOQBDDVVBE-UHFFFAOYSA-N
應用
3-Pyridineboronic acid neopentylglycol ester can be used as a reactant:
- To synthesize N-alkyl-3-boronopyridinium salts by reacting with corresponding alkyl halides.
- In the ruthenium-catalyzed diastereoselective C-H bond α-arylation of cyclic amines.
- To prepare 2-(3-pyridyl)benzoxazole by reacting with benzoxazole via copper-catalyzed oxidative arylation reaction.
- In the Suzuki-Miyaura cross-coupling reaction.
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
Copper-Catalyzed Oxidative Arylation of Heteroarenes under Mild Conditions Using Dioxygen as the Sole Oxidant
Yang F, et al.
Chemistry?A European Journal , 17(23), 6321-6325 (2011)
Nickel/N-Heterocyclic carbene-catalyzed Suzuki-Miyaura type cross-coupling of aryl carbamates
Ohtsuki A, et al.
The Journal of Organic Chemistry, 81(19), 9409-9414 (2016)
Preparation of a series of N-alkyl-3-boronopyridinium halides and study of their stability in the presence of hydrogen peroxide
Karpichev Y, et al.
Central European Journal of Chemistry, 10(4), 1059-1065 (2012)
sp3 C- H bond arylation directed by amidine protecting group: α-arylation of pyrrolidines and piperidines
Pastine SJ, et al.
Journal of the American Chemical Society, 128(44), 14220-14221 (2006)
商品
Suzuki-Miyaura cross-coupling reaction is extensively used in organic chemistry, polymer science, and pharmaceutical industries.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门