推荐产品
等級
technical grade
化驗
90%
折射率
n20/D 1.431
bp
118 °C
密度
0.873 g/mL at 25 °C
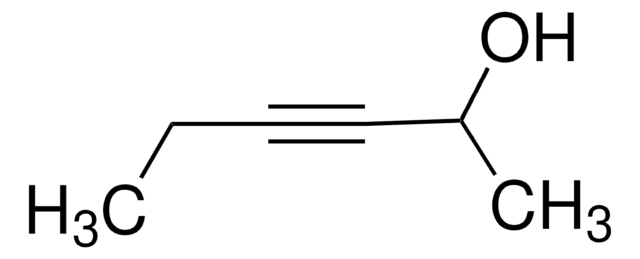
SMILES 字串
CCCC(O)C#C
InChI
1S/C6H10O/c1-3-5-6(7)4-2/h2,6-7H,3,5H2,1H3
InChI 密鑰
LTFTWJYRQNTCHI-UHFFFAOYSA-N
一般說明
1-Hexyn-3-ol is a terminal propargylic alcohol. It undergoes microwave-accelerated coupling-isomerization reaction (MACIR) with (hetero)aryl halides to afford the corresponding enone.
應用
1-Hexyn-3-ol may be used in the synthesis of racemic 3-hydroxy-2-hexanone. It may also be used in the preparation of 1,2,3-triazoles.
訊號詞
Danger
危險分類
Acute Tox. 3 Oral - Eye Dam. 1 - Flam. Liq. 3
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
117.0 °F
閃點(°C)
47.2 °C
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
其他客户在看
Yunfan Zou et al.
Journal of chemical ecology, 41(7), 670-677 (2015-07-08)
We report the identification of a novel pheromone structure from males of the cerambycid beetle Tylonotus bimaculatus Haldeman (Cerambycinae: Hesperophanini), a species native to eastern North America. Volatiles collected from adult males contained (2S,4E)-2-hydroxyoct-4-en-3-one (71%), (3R,4E)-3-hydroxyoct-4-en-2-one (15%), (E)-4-octen-2,3-dione (13%), and
Microwave-Accelerated Coupling-Isomerization Reaction (MACIR)?A General Coupling-Isomerization Synthesis of 1, 3-Diarylprop-2-en-1-ones.
Muller TJJ.
Advanced Synthesis & Catalysis, 348(18), 2565-2570 (2006)
Synthesis of New Carbohydrate Derivatives Via 1, 3-Dipolarcycloaddition Reaction.
Sharba AHK, et al.
Journal of Al-Nahrain University, 14(2), 9S-9S (2011)
The synthesis of 1, 1'-disubstituted bis-cyclopropanes by the reaction of substituted propargylic alcohols with CH 2 I 2?R 3 Al.
Ramazanov IR, et al.
Tetrahedron Letters, 50(29), 4233-4235 (2009)
商品
Alkynes' versatility enables reactions like addition, metathesis, hydroboration, cleavage, coupling, and cycloadditions in synthetic chemistry.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门