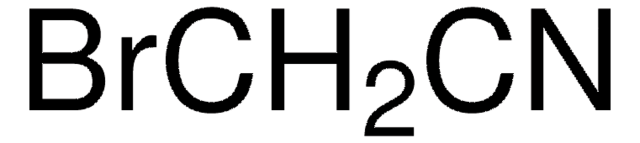所有图片(1)
About This Item
线性分子式:
C6H5OCH2CN
CAS号:
分子量:
133.15
MDL编号:
UNSPSC代码:
12352100
PubChem化学物质编号:
NACRES:
NA.22
推荐产品
方案
98%
折射率
n20/D 1.524 (lit.)
沸点
235-238 °C (lit.)
密度
1.09 g/mL at 25 °C (lit.)
官能团
nitrile
phenoxy
SMILES字符串
N#CCOc1ccccc1
InChI
1S/C8H7NO/c9-6-7-10-8-4-2-1-3-5-8/h1-5H,7H2
InChI key
VLLSCJFPVSQXDM-UHFFFAOYSA-N
应用
Phenoxyacetonitrile may be used in the synthesis of:
- 2,4-dihydroxyphenoxyacetophenones
- methylthio(phenoxy)acetonitrile
- 2,4-diamino-5-(3,4,5-trimethoxyphenoxy)pyrimidine
警示用语:
Warning
危险分类
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral
储存分类代码
10 - Combustible liquids
WGK
WGK 3
闪点(°F)
Not applicable
闪点(°C)
Not applicable
个人防护装备
Eyeshields, Faceshields, Gloves, multi-purpose combination respirator cartridge (US)
A Simple Procedure for the Preparation of (Methyl-and Phenylthio) aryloxyacetonitriles.
Jonczyk A and Muttar EH
Organic preparations and procedures international, 25(6), 690-693 (1993)
Chemistry of modified flavonoids.
Arkhipov VV, et al.
Chemistry of Heterocyclic Compounds, 33(5), 515-519 (1997)
Synthesis of trimethoprim variations. Replacement of methylene by polar groupings.
Stogryn EL.
Journal of Medicinal Chemistry, 15(2), 200-201 (1972)
Reactions of quinoline and 4-chloroquinoline 1-oxides with phenoxyacetonitrile, chloromethylphenylsulfone, and methyl-thiomethyl-p-tolylsulfone.
Hamana M, et al.
Heterocycles, 25, 229-233 (1987)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持