所有图片(1)
About This Item
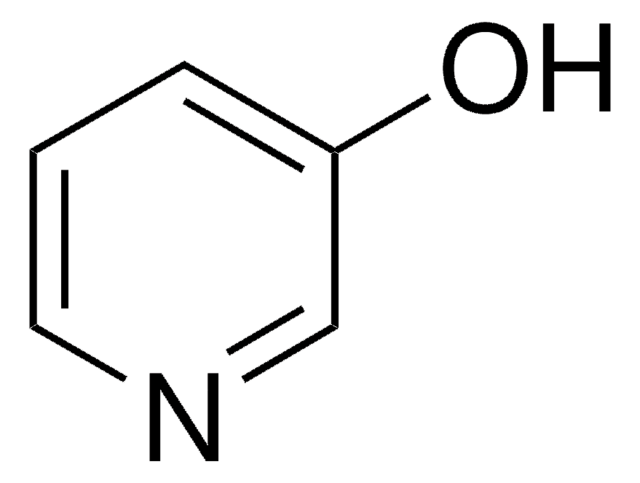
经验公式(希尔记法):
C5H4N2O3
CAS号:
分子量:
140.10
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推荐产品
化驗
98%
mp
285 °C (dec.) (lit.)
SMILES 字串
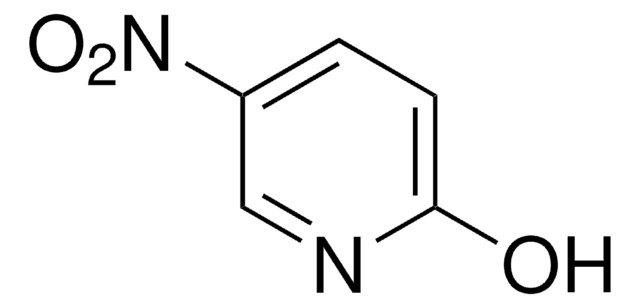
Oc1ccncc1[N+]([O-])=O
InChI
1S/C5H4N2O3/c8-5-1-2-6-3-4(5)7(9)10/h1-3H,(H,6,8)
InChI 密鑰
YUWOLBZMQDGRFV-UHFFFAOYSA-N
一般說明
4-Hydroxy-3-nitropyridine can be synthesized by the nitration of 4-hydroxypyridine.
應用
4-Hydroxy-3-nitropyridine may be used in the synthesis of 4-ethoxy-3-nitropyridine by treating with phosphorus pentachloride (PCl5) followed by ethanol. It may also be used to prepare 4-chloro-3-nitropyridine by treating with PCl5-POCl3 (phosphorus oxychloride).
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
Use of the Graebe-Ullmann reaction in the synthesis of 8-methyl-γ-carboline and isomeric aromatic aza-γ-carbolines.
Alekseev RS, et al.
Chemistry of Heterocyclic Compounds, 48(8), 1235-1250 (2012)
Unambiguous structural assignment of monoanils of 3,4-pyridinediamine via regioselective synthesis.
Dubey PK, et al.
ARKIVOC (Gainesville, FL, United States), 13, 137-144 (2008)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门