所有图片(2)
About This Item
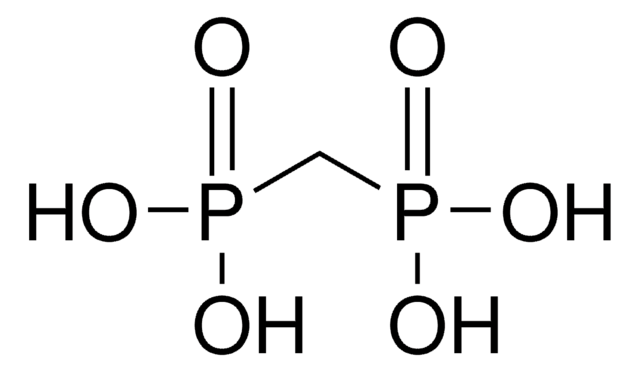
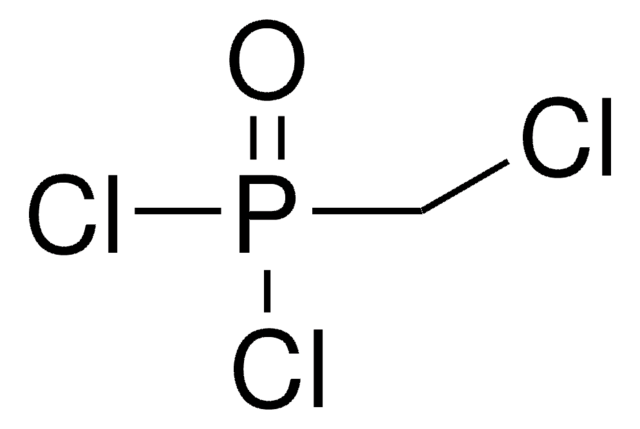
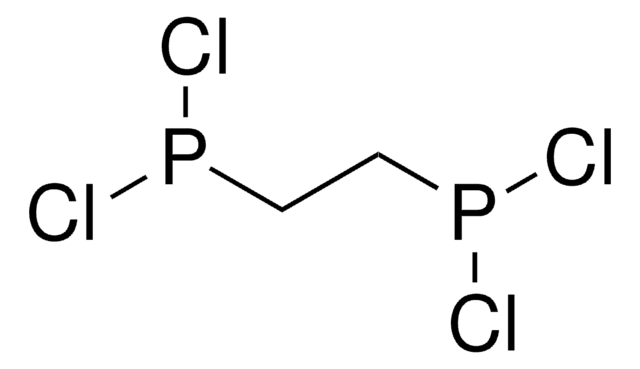
线性分子式:
CH2[P(O)Cl2]2
CAS号:
分子量:
249.78
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推荐产品
化驗
97%
形狀
solid
mp
102-104 °C (lit.)
SMILES 字串
ClP(Cl)(=O)CP(Cl)(Cl)=O
InChI
1S/CH2Cl4O2P2/c2-8(3,6)1-9(4,5)7/h1H2
InChI 密鑰
VRXYCDTWIOCJBH-UHFFFAOYSA-N
一般說明
亚甲基双氯化磷是一种有机磷化合物,常用于膦酰化反应。相比POCl3,反应性更强,反应速率更快。这是由于CH2基的作用(lack of electron back-donation),造成磷原子中心的亲电性更强。
應用
亚甲基双(二氯膦酸)可用于以下研究:
- 麦考酚亚甲基双(膦酸)衍生物的合成。
- 核苷的膦酰化。
- P,P′-亚甲基双膦酸部分酯化物的制备。
- 亚甲基双膦酸对称双酯和对称四酯的合成。
訊號詞
Danger
危險聲明
危險分類
Eye Dam. 1 - Skin Corr. 1B
安全危害
儲存類別代碼
8A - Combustible corrosive hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
其他客户在看
Sanjay Bhattarai et al.
Journal of medicinal chemistry, 63(6), 2941-2957 (2020-02-12)
CD73 inhibitors are promising drugs for the (immuno)therapy of cancer. Here, we present the synthesis, structure-activity relationships, and cocrystal structures of novel derivatives of the competitive CD73 inhibitor α,β-methylene-ADP (AOPCP) substituted in the 2-position. Small polar or lipophilic residues increased
Aviran Amir et al.
The Journal of organic chemistry, 78(2), 270-277 (2012-12-05)
A new transformation of methylene-bis(phosphonic dichloride) into tetrathiobisphosphonate derivatives is reported. The reaction of methylene-bis(phosphonic dichloride) with 1,2-ethanedithiol in bromoform in the presence of AlCl(3) formed methylene-bis(1,3,2-dithiaphospholane-2-sulfide), which gave rise to O,O'-diester-methylenediphosphonotetrathioate analogues 1a-k upon reaction with phenols and alkyl
Kelly S E Tanaka et al.
Bioorganic & medicinal chemistry letters, 20(4), 1355-1359 (2010-01-26)
As therapeutic agents of choice in the treatment of complicated infections, glycopeptide antibiotics are often preferentially used in cases of osteomyelitis, an infection located in bone and notoriously difficult to successfully manage. Yet frequent and heavy doses of these systemically
A direct method for the synthesis of nucleoside 5'-methylenebis (phosphonate) s from nucleosides.
Kalek M, et al.
Tetrahedron Letters, 46(!4), 2417-2421 (2005)
Facile high yielding synthesis of symmetric esters of methylenebisphosphonic acid.
Stepinski DC, et al.
Tetrahedron, 57(41), 8637-8645 (2001)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门