所有图片(1)
About This Item
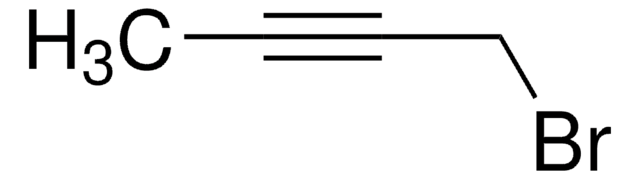
线性分子式:
C2H5C≡CCH2Br
CAS号:
分子量:
147.01
EC號碼:
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推荐产品
化驗
97%
形狀
liquid
折射率
n20/D 1.498 (lit.)
bp
93-94 °C/113 mmHg (lit.)
密度
1.438 g/mL at 25 °C (lit.)
SMILES 字串
CCC#CCBr
InChI
1S/C5H7Br/c1-2-3-4-5-6/h2,5H2,1H3
InChI 密鑰
VDHGRVFJBGRHMD-UHFFFAOYSA-N
一般說明
1-Bromo-2-pentyne is an halogenated hydrocarbon.
應用
1-Bromo-2-pentyne may be employed for the following syntheses:
- stereochemically restricted lactone-type analogs of jasmonic acids, 5-oxa-7-epi-jasmonic acid and 5-oxa-jasmonic acid
- 4,7-decadienal, 4,7-tridecadienal, 5,8-tetradecadienal and 6,9-dodecadienal (all-cis)
- 5-ethyl-4-methylene-6-phenyl-3a,4,7,7a-tetrahydroisobenzofuran-1,3-dione
訊號詞
Warning
危險分類
Eye Irrit. 2 - Flam. Liq. 3 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
105.8 °F - closed cup
閃點(°C)
41 °C - closed cup
其他客户在看
Synthesis of some aliphatic dienals.
Ward JP and Van Dorp DA.
Rec. Trav. Chim., 88(2), 177-184 (1969)
H Toshima et al.
Bioscience, biotechnology, and biochemistry, 64(9), 1988-1992 (2000-10-31)
5-Oxa-7-epi-jasmonic acid and 5-oxa-jasmonic acid, which are stereochemically restricted lactone-type analogues of jasmonic acids, were synthesized via three-component coupling of 2(5H)-furanone, tert-butyl acetate and 1-bromo-2-pentyne. After acidic deprotection of the tert-butyl esters, the (Z)-olefin was introduced by catalytic partial reduction
Synthesis of Cyclic Compounds Having exo-Methylene Groups through the Diels-Alder Reactions of Vinyl Allenes Obtained from Propargyl Bromide and Indium.
Lee K and Lee PH.
Bull. Korean Chem. Soc., 29(2), 487-487 (2008)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门