429066
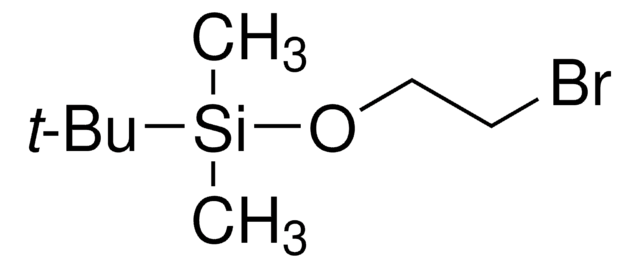
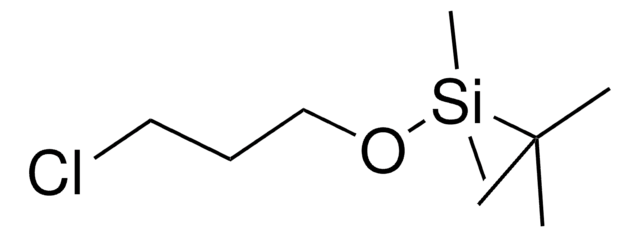
(3-溴丙氧基)-叔丁基二甲基硅烷
97%
别名:
(3-Bromopropoxy)(1,1-dimethylethyl)dimethylsilane, 1-((tert-Butyldimethylsilyl)oxy)-3-bromopropane, 1-Bromo-3-(tert-butyldimethylsiloxy)propane, 1-Bromo-3-[(tert-butyldimethylsilanyl)oxy]propane, 1-Bromo-3-[(tert-butyldimethylsilyl)oxy]propane, 3-((tert-Butyldimethylsilyl)oxy)-1-bromopropane
登录查看公司和协议定价
所有图片(1)
About This Item
线性分子式:
Br(CH2)3OSi(CH3)2C(CH3)3
CAS号:
分子量:
253.25
MDL编号:
UNSPSC代码:
12352100
PubChem化学物质编号:
NACRES:
NA.22
推荐产品
质量水平
方案
97%
表单
liquid
包含
sodium carbonate as stabilizer
折射率
n20/D 1.451 (lit.)
沸点
182 °C (lit.)
密度
1.093 g/mL at 25 °C (lit.)
官能团
bromo
储存温度
2-8°C
SMILES字符串
CC(C)(C)[Si](C)(C)OCCCBr
InChI
1S/C9H21BrOSi/c1-9(2,3)12(4,5)11-8-6-7-10/h6-8H2,1-5H3
InChI key
QGMROEZDWJTIDW-UHFFFAOYSA-N
一般描述
(3-Bromopropoxy)-tert-butyldimethylsilane is a bromo silyl ether.
应用
(3-Bromopropoxy)-tert-butyldimethylsilane may be used to introduce propanol functionality to many pharmaceuticals.
It may be used as an alkylating agent in the synthesis of the following:
It may be used as an alkylating agent in the synthesis of the following:
- N-[2-[N-[3-(tert-butyldimethylsilyloxy)propyl]-N-ethylamino]ethyl]phthalimide
- O-(3-tert-butyldimethylsilyloxypropyl)-N-(tert-butoxycarbonyl)-L-tyrosine methyl ester
- tert-butyldimethyl-[3-(3-methyl-2-nitrophenoxy)propoxy]silane
警示用语:
Warning
危险声明
危险分类
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
靶器官
Respiratory system
储存分类代码
10 - Combustible liquids
WGK
WGK 3
闪点(°F)
185.0 °F - closed cup
闪点(°C)
85 °C - closed cup
其他客户在看
Ramani R Ranatunge et al.
Journal of medicinal chemistry, 47(9), 2180-2193 (2004-04-16)
The synthesis of a series of novel pyrazoles containing a nitrate (ONO(2)) moiety as a nitric oxide (NO)-donor functionality is reported. Their COX-1 and COX-2 inhibitory activities in human whole blood are profiled. Our data demonstrate that pyrazole ring substituents
A concise total synthesis of (+/-)-vigulariol.
J Stephen Clark et al.
Angewandte Chemie (International ed. in English), 46(3), 437-440 (2006-12-06)
Mechanisms of ZnII-activated magnetic resonance imaging agents.
Major JL, et al.
Inorganic Chemistry, 47(22), 10788-10795 (2008)
Margaret M Faul et al.
The Journal of organic chemistry, 69(9), 2967-2975 (2004-04-24)
Synthesis of indolo[6,7-a]pyrrolo[3,4-c]carbazoles 1, a new class of cyclin D1/CDK4 inhibitors, by oxidation of the corresponding aryl indolylmaleimides 2, will be described. Two approaches to the synthesis of 2 were identified that required new methods for the synthesis of 7-substituted
Synthesis, radioiodination and in vivo screening of novel potent iodinated and fluorinated radiotracers as melanoma imaging and therapeutic probes.
Maisonial A, et al.
European Journal of Organic Chemistry, 63, 840-853 (2013)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持