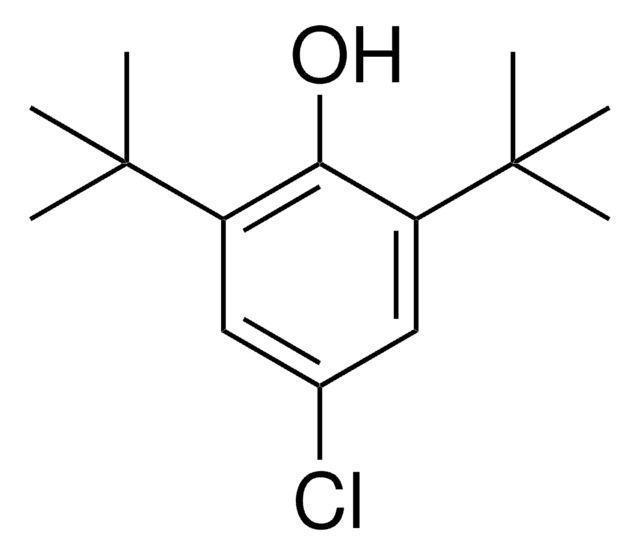
推荐产品
化驗
98%
形狀
solid
mp
78-83 °C (dec.) (lit.)
SMILES 字串
CC(C)(C)c1cc(Br)cc(c1O)C(C)(C)C
InChI
1S/C14H21BrO/c1-13(2,3)10-7-9(15)8-11(12(10)16)14(4,5)6/h7-8,16H,1-6H3
InChI 密鑰
SSQQUEKFNSJLKX-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
4-Bromo-2,6-di-tert-butylphenol is a 4-substituted-2,6-di-tert-butylphenol. It is synthesized by the bromination of 2,6-di-tert-butylphenol. The structure was confirmed by 1H NMR. Its photolysis reaction in benzene solution has been investigated. The photochemical reaction of 4-bromo-2,6-di-tert-butylphenol single crystals doped with 2,6-di-tert-butyl-p-quinone has been studied by EPR spectroscopy.
應用
4-Bromo-2,6-di-tert-butylphenol may be used in the following studies:
- As a terminating comonomer phenol in the phase transfer catalyzed (PTC) polymerization of 4-bromo-2,6-dimethylphenol.
- Synthesis of 1,1-[1,10-decanediylbis(oxy)]bis[(2,6-ditertbutyl-4-bromo)benzene], a monomer, which forms poly(p-phenylenevinylene) derivatives by reaction with 1,10-dibromodecane.
- As a reactant in the synthesis of 2,6-di-tert-butyl-phenolnorbornene (NArOH), a norbornene comonomer bearing an antioxidant hindered phenol.
- As a catalyst with methyl aluminium to form methylaluminum bis(4-bromo-2,6-di-tert-butylphenoxide) (MABR) which may be utilized for the transformation of various epoxides to carbonyl compounds.
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
Effect of donor and acceptor on the mechanism of a photochemical reaction in doped single crystals for radical pairs in 4-bromo-2, 6-di-tert-butylphenol single crystal doped with 2, 6-di-tert-butyl-p-quinone.
Tipikin DS, et al.
Kinetics and Catalysis, 42(2), 246-250 (2001)
An efficient, catalytic procedure for epoxide rearrangement.
Maruoka K, et al.
Tetrahedron Letters, 30(41), 5607-5610 (1989)
Blue light-emitting poly (p-phenylenevinylene) derivatives containing alternating conjugated segments and aliphatic spacers.
Mpallas JG, et al.
Journal of Polymer Science Part A: Polymer Chemistry, 41(8), 1091-1098 (2003)
Phase transfer catalyzed polymerization of 4-bromo-2, 6-dimethylphenol in the presence of a terminating comonomer phenol: 2, 4, 6-tri-tert-butylphenol or 4-bromo-2, 6-di-tert-butylphenol.
Wang JH and Percec V.
Polym. Bull., 25(1), 33-40 (1991)
Photolysis of 4-bromo-2, 6-di-tert-butylphenol in benzene solution.
Lappin GR and Zannucci JS.
Tetrahedron Letters, 10(58), 5085-5087 (1969)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门