所有图片(1)
About This Item
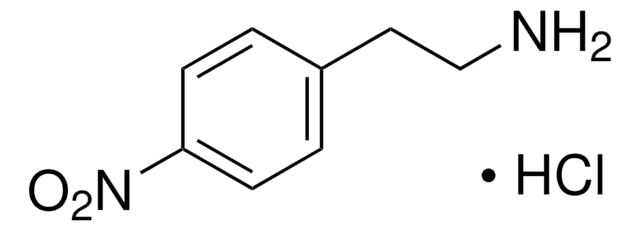
线性分子式:
O2NC6H4CH(CH3)NH2 · HCl
CAS号:
分子量:
202.64
MDL號碼:
分類程式碼代碼:
12352116
PubChem物質ID:
NACRES:
NA.22
推荐产品
化驗
97%
形狀
powder
光學活性
[α]25/D −6.5°, c = 1 in 0.05 M NaOH
mp
248-250 °C (lit.)
SMILES 字串
Cl.C[C@H](N)c1ccc(cc1)[N+]([O-])=O
InChI
1S/C8H10N2O2.ClH/c1-6(9)7-2-4-8(5-3-7)10(11)12;/h2-6H,9H2,1H3;1H/t6-;/m0./s1
InChI 密鑰
CZQQGVFHLSBEDV-RGMNGODLSA-N
正在寻找类似产品? 访问 产品对比指南
應用
(S)-α-Methyl-4-nitrobenzylamine hydrochloride can be used as:
- A fluorescence-quenching guest in the determination of chiral recognition capabilities of binaphthocrown ether and polythiophene complex.
- A starting material in the synthesis of chiral cyclopalladated complexes with applications in the resolution of racemic compounds.
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
Polymer-based supramolecular sensing and application to chiral photochemistry
Fukuhara G
Polymer Journal, 47(10), 649-655 (2015)
The first orthopalladation of a primary nitrobenzylamine. Synthesis of chiral cyclopalladated complexes derived from (S)-α-methyl-4-nitrobenzylamine
Vicente J, et al.
J. Chem. Soc., Dalton Trans., 47(15), 2535-2539 (1995)
Gaku Fukuhara et al.
Chemical communications (Cambridge, England), 48(11), 1641-1643 (2011-12-15)
Chiral recognition abilities of the title host for (R)- and (S)-α-methyl-4-nitrobenzylamine were examined in the ground and excited states to give a relative affinity (K(R)/K(S)) of 2.16 by spectral titration and a relative rate constant (k(R)/k(S)) of 2.23 by fluorescence
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门