所有图片(1)
About This Item
经验公式(希尔记法):
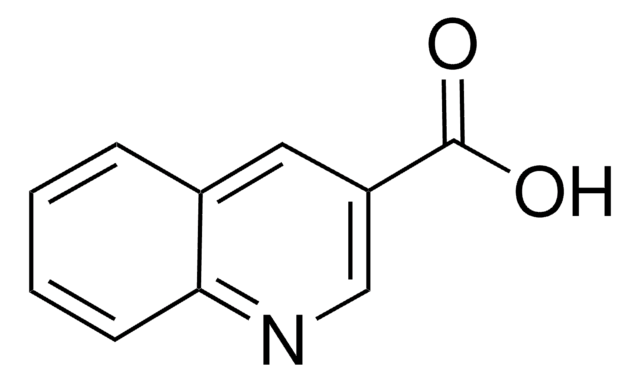
C24H21NO4
CAS号:
分子量:
387.43
MDL编号:
UNSPSC代码:
12352005
PubChem化学物质编号:
NACRES:
NA.22
推荐产品
方案
99%
旋光性
[α]25/D +66°, c = 5.5 in methylene chloride
mp
205-207 °C (lit.)
储存温度
2-8°C
SMILES字符串
O=C1CN([C@@H]([C@@H](O1)c2ccccc2)c3ccccc3)C(=O)OCc4ccccc4
InChI
1S/C24H21NO4/c26-21-16-25(24(27)28-17-18-10-4-1-5-11-18)22(19-12-6-2-7-13-19)23(29-21)20-14-8-3-9-15-20/h1-15,22-23H,16-17H2/t22-,23+/m1/s1
InChI key
HECRUWTZAMPQOS-PKTZIBPZSA-N
应用
(2S,3R)-(+)-N-Z-6-oxo-2,3-diphenylmorpholine can be used as a starting material for the synthesis of:
- α-Amino-β-silyloxy-ester, a key intermediate for the preparation of antimalarial drug quinine and its analogs.
- R, R-Formylglycine dimethylacetal, a key intermediate for the preparation of tuberculostatic compound capreomycin IB.
- 2R,5R,6S-2-(Methoxycarbonylmethyl)-5,6-diphenylmorpholine hydrochloride, a key intermediate for the preparation of an antibiotic (+)-negamycin.
警示用语:
Warning
危险声明
危险分类
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
靶器官
Respiratory system
储存分类代码
11 - Combustible Solids
WGK
WGK 3
闪点(°F)
Not applicable
闪点(°C)
Not applicable
个人防护装备
dust mask type N95 (US), Eyeshields, Gloves
Asymmetric synthesis of (2S, 3R)-capreomycidine and the total synthesis of capreomycin IB
DeMong DE and Williams RM
Journal of the American Chemical Society, 125(28), 8561-8565 (2003)
Asymmetric synthesis of (+)-negamycin
Jain RP and Williams RM
The Journal of Organic Chemistry, 67(18), 6361-6365 (2002)
Synthetic studies on quinine: quinuclidine construction via a Ketone Enolate regio-and diastereoselective Pd-mediated allylic alkylation
Johns DM, et al.
Organic Letters, 8(18), 4051-4054 (2006)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门![苯并[g]异喹啉-5,10-二酮 99%](/deepweb/assets/sigmaaldrich/product/structures/484/029/288c4a9d-19c2-4b51-82c1-f43b50ea05b0/640/288c4a9d-19c2-4b51-82c1-f43b50ea05b0.png)