所有图片(1)
About This Item
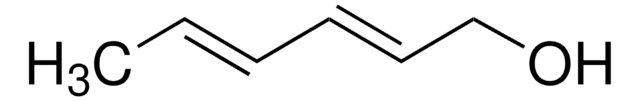
线性分子式:
CH2=CHCH(OH)CH=CH2
CAS号:
分子量:
84.12
Beilstein:
1735809
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推荐产品
化驗
≥96%
形狀
liquid
包含
0.4% hydroquinone as stabilizer
折射率
n20/D 1.445 (lit.)
bp
115-116 °C (lit.)
密度
0.865 g/mL at 25 °C (lit.)
SMILES 字串
OC(C=C)C=C
InChI
1S/C5H8O/c1-3-5(6)4-2/h3-6H,1-2H2
InChI 密鑰
ICMWSAALRSINTC-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
不对称环氧化反应的原料。
在合成氨基取代的二烯时用作底物,其中通过磺酰胺亲核试剂进行铋催化 SNi 醇置换。
天然产物合成中的有用结构单元。
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral - Aquatic Chronic 2 - Flam. Liq. 3
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
89.6 °F - closed cup
閃點(°C)
32 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
其他客户在看
Chin. J. Chem., 9, 381-381 (1991)
Masanori Imai et al.
The Journal of organic chemistry, 69(4), 1144-1150 (2004-02-14)
Intermolecular hydroacylation between salicylaldehydes 1, 26-40 and 1,4-penta- or 1,5-hexadienes 4-13 by Rh-catalyst proceeded under mild reaction conditions to give a mixture of iso- and normal-hydroacylated products 14-25, 41-55, and 57-60. In the hydroacylation reaction, chelation of both salicylaldehyde and
Tetrahedron Asymmetry, 4, 1533-1533 (1993)
P Andrew Evans et al.
Journal of the American Chemical Society, 125(48), 14702-14703 (2003-12-04)
The enantioselective total synthesis of the annonaceous acetogenin (-)-mucocin (1) was accomplished using a triply convergent 12-step sequence (longest linear sequence) in 13.6% overall yield. This represents the first application of the temporary silicon-tethered (TST) ring-closing metathesis (RCM) cross-coupling reaction
Bismuth-catalyzed direct substitution of the hydroxy group in alcohols with sulfonamides, carbamates, and carboxamides.
Hongbo Qin et al.
Angewandte Chemie (International ed. in English), 46(3), 409-413 (2006-12-06)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门