所有图片(1)
About This Item
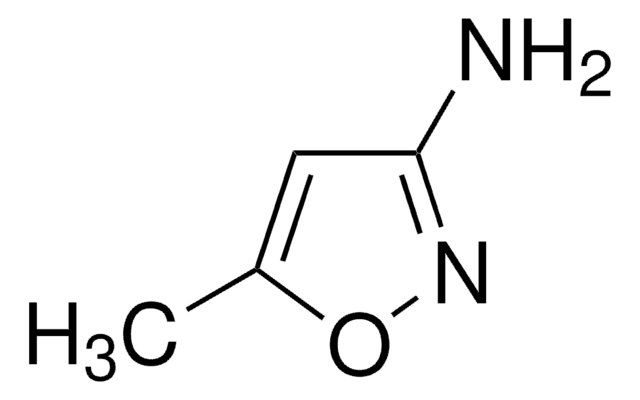
经验公式(希尔记法):
C4H6N2O
CAS号:
分子量:
98.10
EC 号:
MDL编号:
UNSPSC代码:
12352100
PubChem化学物质编号:
NACRES:
NA.22
推荐产品
方案
98%
mp
85-87 °C (lit.)
SMILES字符串
Cc1cc(N)on1
InChI
1S/C4H6N2O/c1-3-2-4(5)7-6-3/h2H,5H2,1H3
InChI key
FNXYWHTZDAVRTB-UHFFFAOYSA-N
一般描述
5-氨基-3-甲基异噁唑可与α,β-不饱和酮反应形成相应的异噁唑[5,4-b]吡啶。
警示用语:
Warning
危险声明
危险分类
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
靶器官
Respiratory system
储存分类代码
11 - Combustible Solids
WGK
WGK 3
闪点(°F)
Not applicable
闪点(°C)
Not applicable
个人防护装备
dust mask type N95 (US), Eyeshields, Gloves
Wafaa S Hamama et al.
Archiv der Pharmazie, 345(6), 468-475 (2012-03-03)
The reaction of 5-amino-3-methylisoxazole with appropriate α,β-unsaturated ketones gave the corresponding isoxazolo[5,4-b]pyridines. Treatment of 1 with 2,6-dibenzylidenecyclohexanone or 2-benzylidenedimedone afforded the corresponding isoxazolo[5,4-b]quinoline derivatives. 4,6,8,9-Tetrahydroisoxazolo[5,4-b]quinolin-5-one derivative was also obtained by multicomponent condensation reaction of 1 with dimedone and benzaldehyde. Heterocyclic
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系客户支持