所有图片(1)
About This Item
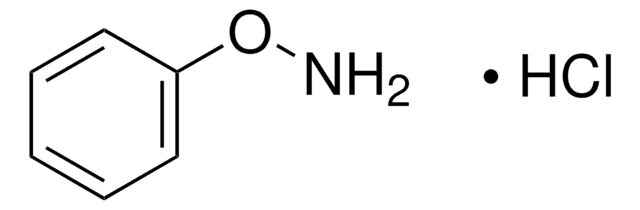
线性分子式:
C6H5CH2NHOH · HCl
CAS号:
分子量:
159.61
Beilstein:
507948
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推荐产品
等級
puriss.
品質等級
化驗
≥99.0% (AT)
形狀
solid
mp
~105 °C
108-110 °C (lit.)
SMILES 字串
Cl.ONCc1ccccc1
InChI
1S/C7H9NO.ClH/c9-8-6-7-4-2-1-3-5-7;/h1-5,8-9H,6H2;1H
InChI 密鑰
YSNXOQGDHGUKCZ-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
N-Benzylhydroxylamine hydrochloride was used in the synthesis of sugar derived nitrones. It was used as starting reagent in the synthesis of fluoro isoxazoline and isoxazolidine derivatives using flouro nitrone.
生化/生理作用
N-Benzylhydroxylamine is a potential pharmacological agent in the prevention and progression of acrolein-induced damage to the retinal pigment epithelium.
其他說明
从羰基化合物合成硝酮以及与烯烃的 [2+3]-环加成生成异噁唑烷(通用中间体);烯丙基酯的钯催化羟胺化反应。
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
B.J. Wakefield
Sci. Synth., 11, 229-229 (2002)
T. Kawakami et al.
Bulletin of the Chemical Society of Japan, 73, 2423-2423 (2000)
Ionic liquid mediated synthesis of some novel fluoro isoxazolidine and isoxazoline derivatives using N-benzyl fluoro nitrone via cycloaddition reaction and their antimicrobial activities.
Chakraborty B, et al.
Indian J. Chem. B, 52(10), 1342-1351 (2013)
R Herrera et al.
The Journal of organic chemistry, 66(4), 1252-1263 (2001-04-21)
Captodative olefins 1-acetylvinyl carboxylates proved to be highly regioselective dipolarophiles in 1,3-dipolar cycloadditon to propionitrile oxide, arylphenylnitrile imines, diazoalkanes, and nitrones to yield the corresponding 5-substituted heterocycles. The addition of the latter was also stereoselective, being slightly susceptible to steric
Synthesis of trihydroxy quinolizidine alkaloids: 1, 3-addition reaction of allylmagnesium bromide to a sugar nitrone.
Dhavale DD, et al.
Tetrahedron, 60(13), 3009-3016 (2004)
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门