C7757
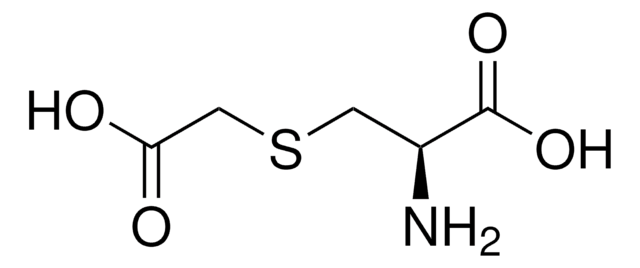
S-Carboxymethyl-L-cysteine
About This Item
Productos recomendados
Ensayo
>98% (TLC)
Nivel de calidad
Formulario
powder
técnicas
cell culture | mammalian: suitable
color
white
mp
200 °C
temp. de almacenamiento
2-8°C
cadena SMILES
N[C@@H](CSCC(O)=O)C(O)=O
InChI
1S/C5H9NO4S/c6-3(5(9)10)1-11-2-4(7)8/h3H,1-2,6H2,(H,7,8)(H,9,10)/t3-/m0/s1
Clave InChI
GBFLZEXEOZUWRN-VKHMYHEASA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Acciones bioquímicas o fisiológicas
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 2
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Importance and uses of cysteine in serum-free eukaryotic, including hybridoma and Chinese Hamster Ovary (CHO) cell, cultures
Importance and uses of cysteine in serum-free eukaryotic, including hybridoma and Chinese Hamster Ovary (CHO) cell, cultures
Importance and uses of cysteine in serum-free eukaryotic, including hybridoma and Chinese Hamster Ovary (CHO) cell, cultures
Importance and uses of cysteine in serum-free eukaryotic, including hybridoma and Chinese Hamster Ovary (CHO) cell, cultures
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico