B9385
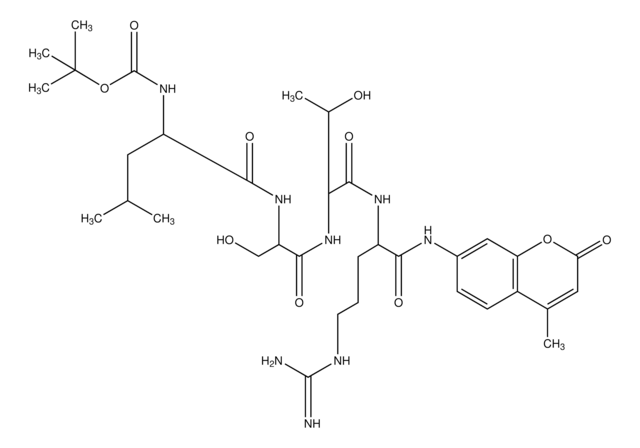
Boc-Val-Pro-Arg-7-amido-4-methylcoumarin hydrochloride
About This Item
Productos recomendados
Ensayo
≥98% (TLC)
Nivel de calidad
Formulario
powder
solubilidad
water: 20 mg/mL, clear, colorless
temp. de almacenamiento
−20°C
cadena SMILES
Cl.CC(C)C(NC(=O)OC(C)(C)C)C(=O)N1CCCC1C(=O)NC(CCCNC(N)=N)C(=O)Nc2ccc3C(C)=CC(=O)Oc3c2
InChI
1S/C31H45N7O7.ClH/c1-17(2)25(37-30(43)45-31(4,5)6)28(42)38-14-8-10-22(38)27(41)36-21(9-7-13-34-29(32)33)26(40)35-19-11-12-20-18(3)15-24(39)44-23(20)16-19;/h11-12,15-17,21-22,25H,7-10,13-14H2,1-6H3,(H,35,40)(H,36,41)(H,37,43)(H4,32,33,34);1H
Clave InChI
ROQGJTZGLVLVIN-UHFFFAOYSA-N
Descripción general
Aplicación
- as a fluorogenic substrate for thrombin in inhibitory assay with salivary protein cE5[2]
- as a fluorogenic substrate for thrombin in inhibitory assay with recombinant E. nipponicum serpin[3]
- as a substrate in proteinase assay with hemolymph protein[4]
Envase
Sustratos
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Protocolos
Thrombin is an endolytic serine protease that selectively cleaves the Arg–Gly bonds of fibrinogen to form fibrin and release fibrinopeptides A and B.
Active Filters
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico