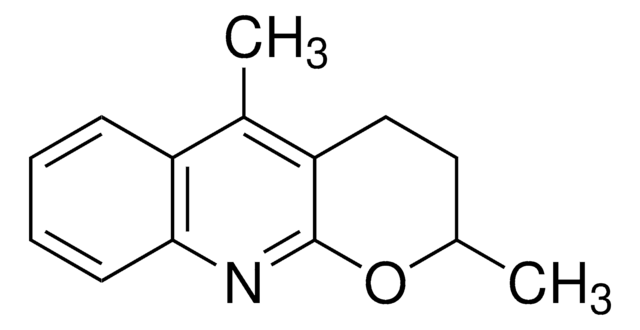
ALD00606
Wang−Yu non-directed C−H functionalization ligand
95%
About This Item
Productos recomendados
assay
95%
form
powder or crystals
reaction suitability
reaction type: C-C Bond Formation
reagent type: catalyst
reaction type: C-H Activation
mp
145 °C
storage temp.
−20°C
Categorías relacionadas
Application
related product
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Contenido relacionado
The Yu program centers around the discovery of catalytic carbon–carbon and carbon–heteroatom bond forming reactions based on C–H activation. Target transformations are selected to enable 1) the use of simple and abundant starting materials such as aliphatic acids, amines and alcohols, and 2) disconnections that drastically shorten the synthesis of a drug molecule or a major class of biologically active compounds.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico

![1-clorometil-4-fluoro-1,4-diazoniabiciclo[2,2.2]octano bis(tetrafluoroborato) >95% in F+ active](/deepweb/assets/sigmaaldrich/product/structures/206/487/53d52ee5-ef71-4e9a-9bc8-938b68b98d5d/640/53d52ee5-ef71-4e9a-9bc8-938b68b98d5d.png)