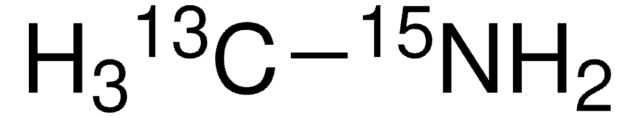490288
Methylamine-15N hydrochloride
98 atom % 15N
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
CH315NH2·HCl
Número de CAS:
Peso molecular:
68.51
Número MDL:
Código UNSPSC:
12352116
ID de la sustancia en PubChem:
NACRES:
NA.12
Productos recomendados
pureza isotópica
98 atom % 15N
Nivel de calidad
Formulario
solid
mp
232-234 °C (lit.)
cambio de masa
M+1
cadena SMILES
Cl.C[15NH2]
InChI
1S/CH5N.ClH/c1-2;/h2H2,1H3;1H/i2+1;
Clave InChI
NQMRYBIKMRVZLB-CGOMOMTCSA-N
Categorías relacionadas
Envase
This product may be available from bulk stock and can be packaged on demand. For information on pricing, availability and packaging, please contact Stable Isotopes Customer Service.
Palabra de señalización
Warning
Frases de peligro
Clasificaciones de peligro
Acute Tox. 4 Oral
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Sheeza Khan et al.
Protein and peptide letters, 20(1), 61-70 (2012-06-08)
Kidney cells of animals including human and marine invertebrates contain high amount of the protein denaturant, urea. Methylamine osmolytes are generally believed to offset the harmful effects of urea on proteins in vitro and in vivo. In this study we
Yongqian Zhang et al.
Analytica chimica acta, 752, 106-111 (2012-10-30)
Both endogenous and exogenous methylamine have been found to be involved in many human disorders. The quantitative assessment of methylamine has drawn considerable interest in recent years. Although there have been many papers about the determination of methylamine, only a
Nicolas Fleury-Brégeot et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 18(31), 9564-9570 (2012-07-07)
Ammoniomethyl trifluoroborates are very powerful reagents that can be used to access biologically relevant aryl- and heteroaryl-methylamine motifs via Suzuki-Miyaura cross-couplings. Until now, this method was limited to the production of tertiary and primary amines. The synthesis of a large
Chaehyuk Ko et al.
The journal of physical chemistry. B, 117(1), 316-325 (2012-12-15)
Proton-coupled electron transfer can occur through concerted (electron-proton transfer, EPT) or sequential mechanisms, but this distinction becomes less well-defined for photoinduced reactions. These issues have been examined with transient absorption experiments on a hydrogen-bonded complex consisting of p-nitrophenylphenol and t-butylamine.
Frank Weinhold
Journal of computational chemistry, 33(30), 2440-2449 (2012-07-28)
We have developed a "Natural Bond Critical Point" (NBCP) module for the natural bond orbital (NBO) program that allows mutual analysis of NBO-based versus Bader-type quantum theory of atoms in molecules (QTAIM) topological descriptors of chemical bonding interactions. Conventional QTAIM
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico