M80806
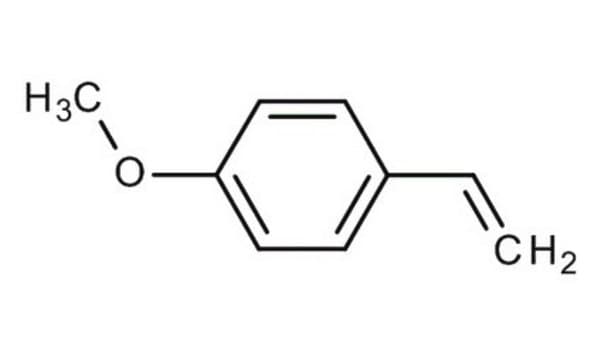
4-Methylstyrene
96%, contains 3,5-di-tert-butylcatechol as inhibitor
Synonym(s):
4-Vinyltoluene
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
CH3C6H4CH=CH2
CAS Number:
Molecular Weight:
118.18
Beilstein:
1209317
EC Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
vapor pressure
<1 mmHg ( 20 °C)
Quality Level
Assay
96%
form
liquid
autoignition temp.
959 °F
contains
3,5-di-tert-butylcatechol as inhibitor
expl. lim.
5.3 %
refractive index
n20/D 1.542 (lit.)
bp
170-175 °C (lit.)
density
0.897 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
Cc1ccc(C=C)cc1
InChI
1S/C9H10/c1-3-9-6-4-8(2)5-7-9/h3-7H,1H2,2H3
InChI key
JLBJTVDPSNHSKJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
4-Methylstyrene is a molecule with an extended Π conjugation. The threefold symmetric torsional potential of 4-methylstyrene has been investigated. Polymerization of 4-methylstyrene by employing a half-metallocene type catalytic system composed of (trimethyl)pentamethylcyclopentadienyltitanium (Cp*TiMe3), trioctylaluminum (AlOct3), and tris(pentafluorophenyl)borane [B(C6F5)3] has been reported. Palladium-catalyzed Heck coupling of chlorobenzene with 4-methylstyrene has been investigated.
Application
4-Methylstyrene was employed as Π ligand in the preparation of cationic, two-coordinate triphenylphosphine-gold(I)-Π complexes.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 2 - Asp. Tox. 1 - Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
113.0 °F
Flash Point(C)
45 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Rachel E M Brooner et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 19(25), 8276-8284 (2013-04-18)
Cationic, two-coordinate triphenylphosphine-gold(I)-π complexes of the form [(PPh₃)Au(π ligand)]⁺SbF₆⁻ (π ligand=4-methylstyrene, 1∙SbF₆), 2-methyl-2-butene (3∙SbF₆), 3-hexyne (6∙SbF₆), 1,3-cyclohexadiene (7∙SbF₆), 3-methyl-1,2-butadiene (8∙SbF₆), and 1,7-diphenyl-3,4-heptadiene (10∙SbF₆) were generated in situ from reaction of [(PPh₃)AuCl], AgSbF₆, and π ligand at -78 °C and were
Rajeev K Sinha et al.
The Journal of chemical physics, 124(14), 144316-144316 (2006-04-22)
To understand the effect of the para position vinyl group substitution in toluene on methyl torsion, we investigated 4-methylstyrene, a benchmark molecule with an extended pi conjugation. The assignment for a 33 cm(-1) band in the excitation spectrum to the
Syndiospecific living polymerization of 4-methylstyrene and styrene with (trimethyl) pentamethylcyclopentadienyltitanium/tris (pentafluorophenyl) borane/trioctylaluminum catalytic system.
Kawabe M and Murata M.
Journal of Polymer Science Part A: Polymer Chemistry, 39(21), 3692-3706 (2001)
An efficient palladium-catalyzed Heck coupling of aryl chlorides with alkenes.
Yi C and Hua R.
Tetrahedron Letters, 47(15), 2573-2576 (2006)
T Kühler
Xenobiotica; the fate of foreign compounds in biological systems, 14(5), 417-428 (1984-05-01)
N-Acetyl-L-cysteine was reacted with 2-(2-, 3-, or 4-methylphenyl)-oxiranes to give mixtures of the two possible regio isomers N-acetyl-S-[1-(2-, 3-, or 4-methylphenyl)-2-hydroxyethyl]-L-cysteine and N-acetyl-S-[2-(2-, 3-, or 4-methylphenyl)-2-hydroxyethyl]-L-cysteine, respectively. These were isolated in pure form by h.p.l.c.. The diastereomers were characterized by
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service