C11006
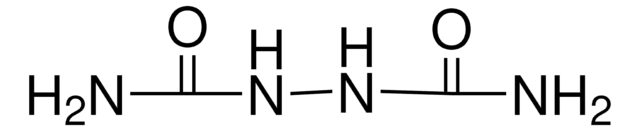
Carbohydrazide
98%
Synonym(s):
1,3-Diaminourea, Carbodihydrazide, Carbonohydrazide, N,N′-Diaminourea
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
CO(NHNH2)2
CAS Number:
Molecular Weight:
90.08
Beilstein:
1747069
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
crystals
mp
150-153 °C (lit.)
SMILES string
NNC(=O)NN
InChI
1S/CH6N4O/c2-4-1(6)5-3/h2-3H2,(H2,4,5,6)
InChI key
XEVRDFDBXJMZFG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Carbohydrazide can be used:
- As an oxygen scavenger.
- In the synthesis of polydentate Schiff base ligands with various aldehydes and ligands by condensation.
- In the synthesis of trifluoromethyl-containing (E)-N′-arylidene-1H-pyrazole-1-carbohydrazides by cyclocondensation reaction which shows antioxidant and antimicrobial properties.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 2 - Skin Irrit. 2 - Skin Sens. 1
Supplementary Hazards
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Marta Kisiel et al.
Journal of controlled release : official journal of the Controlled Release Society, 162(3), 646-653 (2012-08-21)
Understanding the influence of formulation and storage conditions on rhBMP-2 bioactivity is extremely important for its clinical application. Reports in the literature show that different research groups employ different parameters such as formulation conditions, storage, doses for in vivo applications
Ameen Ali Abu-Hashem et al.
Acta pharmaceutica (Zagreb, Croatia), 60(3), 311-323 (2010-12-08)
2-Amino-5-acetyl-4-methyl-thiophene-3-carboxylic acid ethyl ester (1) and 5-acetyl-2-amino-4-methylthiophene-3-carbohydrazide (2) were synthesized and used as starting materials for the synthesis of new series of 1-(5-amino-4-(3,5-dimethyl-1H-pyrazole-1-carbonyl)-3-methylthiophen-2-yl) ethanone (3a), 1-(5-amino-4-(4-chloro-3,5-dimethyl-1H-pyrazole-1-carbonyl)-3-methylthiophen-2-yl) ethanone (3b), 1-(4-methyl-2-amino-5-acetylthiophene-3-carbonyl)pyrazolidine-3,5-dione (4), (Z)-N'-(4-methyl-2-amino-5-acetylthiophene-3-carbonyl) formohydrazonic acid (5a), (Z)-ethyl-N'-4-methyl-2-amino-5-acetylthiophene-3-carbonylformo hydrazonate (5b), 6-acetyl-3-amino-2,5-dimethylthieno[2,3-d]pyrimidin-4(3H)-one (8), 5-methyl-3-amino-2-mercapto-6-acetylthieno
Synthesis, structure and reactivity of zirconium (IV), vanadium (IV), cobalt (II), nickel (II) and copper (II) complexes derived from carbohydrazide schiff base ligands.
Warad, DU, et al.
Indian Journal of Chemistry, 39A(10), 415-420 (2000)
Mateusz Wierzbicki et al.
Nanoscale research letters, 14(1), 320-320 (2019-10-12)
Antibacterial surfaces coated with nanomaterials, including silver nanoparticles, are considered effective alternative antimicrobial agents that can be used instead of antibiotics and chemical agents. However, reports of the potential toxicity of these materials raise questions about the safety of their
Alessandro K Jordão et al.
Bioorganic & medicinal chemistry, 19(18), 5605-5611 (2011-08-16)
Tuberculosis treatment remains a challenge that requires new antitubercular agents due to the emergence of multidrug-resistant Mycobacterium strains. This paper describes the synthesis, the antitubercular activity and the theoretical analysis of N-substituted-phenylamino-5-methyl-1H-1,2,3-triazole-4-carbohydrazides (8a-b, 8e-f, 8i-j and 8n-o) and new analogues
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service


![Calix[4]arene-25,26,27,28-tetrol 95%](/deepweb/assets/sigmaaldrich/product/structures/198/765/9972559b-b50f-4745-b1fd-9a468e47ef55/640/9972559b-b50f-4745-b1fd-9a468e47ef55.png)