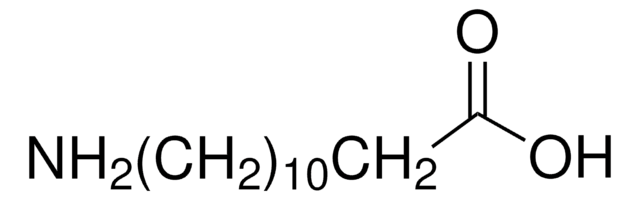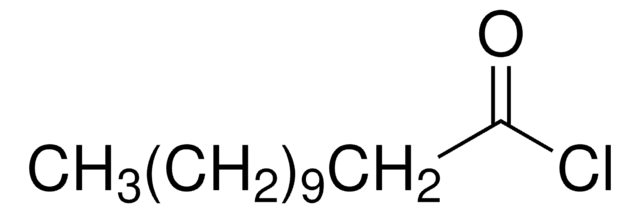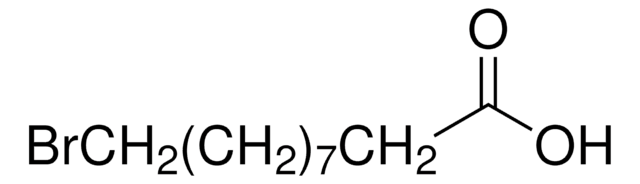A82605
11-Aminoundecanoic acid
97%
Synonym(s):
Aminoundecanoic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
NH2(CH2)10CO2H
CAS Number:
Molecular Weight:
201.31
Beilstein:
1767291
EC Number:
MDL number:
UNSPSC Code:
12352106
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
powder
reaction suitability
reaction type: solution phase peptide synthesis
color
white
mp
188-191 °C (lit.)
application(s)
peptide synthesis
SMILES string
NCCCCCCCCCCC(O)=O
InChI
1S/C11H23NO2/c12-10-8-6-4-2-1-3-5-7-9-11(13)14/h1-10,12H2,(H,13,14)
InChI key
GUOSQNAUYHMCRU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
11-Aminoundecanoic acid also known as aminoundecanoic acid, is utilized in solution phase peptide synthesis. It is also a monomer precursor for nylon-11.
Application
11-Aminoundecanoic acid can be used as a linker to synthesize amide-linked linear guanosine dimer.
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Development of Minimal Diguanosinyl Motif toward RNA G-Quadruplex-Like Structures in Solution
Chembiochem, 21, 1837-1842 (2020)
Syntheses of 12-aminododecanoic and 11-aminoundecanoic acids from vernolic acid
Journal of the American Oil Chemists' Society, 74, 531-538 (1997)
M Marastoni et al.
European journal of medicinal chemistry, 35(6), 593-598 (2000-07-25)
The latent membrane protein 2 (LMP2) is expressed in EBV-associated tumours. LMP2 is a target of HLA-A2 restricted EBV-specific CTL responses and consequently it may represent a good target for specific CTL-based immunotherapies. However, the efficacy of such therapy is
Liling Zeng et al.
Nano letters, 5(10), 2001-2004 (2005-10-13)
Carboxylic acid-functionalized SWNTs prepared via the reaction of an amino acid, NH2(CH2)nCO2H, with fluoronanotubes show similar levels of sidewall functionalization; however, the solubility in water is controlled by the length of the hydrocarbon side chain (i.e., n). The 6-aminohexanoic acid
E J Matthews
Environmental health perspectives, 101 Suppl 2, 311-318 (1993-07-01)
A co-culture clonal survival assay was developed to measure the cytotoxicity of test chemical treatments to BALB/c-3T3 cells because the standard clonal survival assay using 200 wild type (WT) cells frequently overestimates chemical cytotoxicity when compared with identical treatment doses
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service