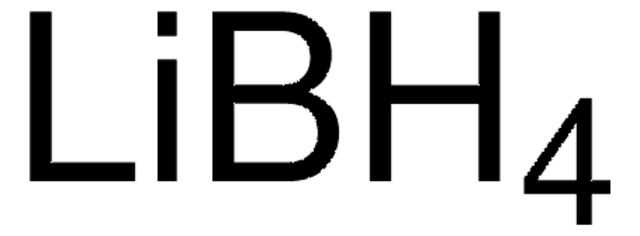62460
Lithium borohydride
≥95.0%
Synonym(s):
Lithium boron hydride, Lithium hydroborate
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
LiBH4
CAS Number:
Molecular Weight:
21.78
EC Number:
MDL number:
UNSPSC Code:
26111700
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥95.0%
form
solid
reaction suitability
reagent type: reductant
mp
275 °C (dec.)
density
0.666 g/mL at 25 °C (lit.)
SMILES string
[Li+].[BH4-]
InChI
1S/BH4.Li/h1H4;/q-1;+1
InChI key
UUKMSDRCXNLYOO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Lithium borohydride is a general reducing agent commonly used to reduce aldehydes, ketones esters, lactones, and epoxides. It catalyzes hydroboration of alkenes. It is also used in the preparation of other borohydrides such as aluminum borohydride.
Other Notes
Review; Smooth reduction of esters; Reduction of acids and other functional groups with LiBH4/Me3SiCl
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Eye Dam. 1 - Skin Corr. 1B - Water-react 1
Storage Class Code
4.3 - Hazardous materials which set free flammable gases upon contact with water
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
H.C. Brown et al.
The Journal of Organic Chemistry, 47, 1604-1604 (1982)
Lithium Borohydride.
eEROS (Encyclopedia of Reagents for Organic Synthesis) (2001)
Lithium borohydride-catalyzed selective reduction of the carbonyl group of conjugated and unconjugated alkenones with borane in tetrahydrofuran.
Arase, Akira et al.
Journal of the Chemical Society. Chemical Communications, 51(7), 855-856 (1994)
The Preparation of Other Borohydrides by Metathetical Reactions Utilizing the Alkali Metal Borohydrides1.
Schlesinger, HI et al.
Journal of the American Chemical Society, 75(1), 209-213 (1953)
LiBH 4-promoted hydroboration of alkenes with 1, 3, 2-benzodioxaborole.
Arase, Akira et al.
Journal of the Chemical Society. Chemical Communications, 51(4), 205-206 (1991)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service