578045
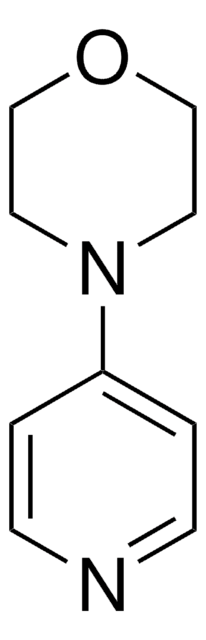
4-Morpholinopiperidine
98%
Synonym(s):
4-(4-Piperidinyl) morpholine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H18N2O
CAS Number:
Molecular Weight:
170.25
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
bp
100-115 °C/0.15-0.20 mmHg (lit.)
mp
40-43 °C (lit.)
functional group
ether
SMILES string
C1CC(CCN1)N2CCOCC2
InChI
1S/C9H18N2O/c1-3-10-4-2-9(1)11-5-7-12-8-6-11/h9-10H,1-8H2
InChI key
YYBXNWIRMJXEQJ-UHFFFAOYSA-N
Application
4-Morpholinopiperidine may be used to synthesize 2-(4-morpholinopiperidin-1-yl)-5-nitrobenzonitrile and 9-bromo-6,6-dimethyl-8-(4-morpholin-4-yl-piperidin-1-yl)-11-oxo-6,11-dihydro-5H-benzo[b]carbazole-3-carbonitrile.
Reactant for synthesis of:
- Selective adenosine A2A receptor antagonists
- Antidepressents
- Small molecules that restore E-cadherin expression and reduce invasion in colorectal carcinoma cells
- Orally bioavailable P2Y12 antagonists for inhibition of platelet aggregation
- Quinoline derivatives with antimicrobial activity
- Antimalarials
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
"A new phenylpyrazoleanilide, y-320, inhibits interleukin 17 production and ameliorates collagen-induced arthritis in mice and cynomolgus monkeys"
Ushio H, et al.
Pharmaceuticals (Basel, Switzerland), 7(01), 1-17 (2013)
"Design and synthesis of a highly selective, orally active and potent anaplastic lymphoma kinase inhibitor (CH5424802)"
Kinoshita K, et al.
Bioorganic & Medicinal Chemistry, 20(03), 1271-1280 (2012)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service