142360
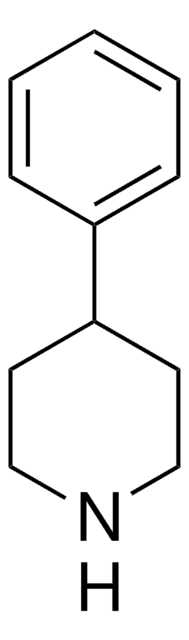
4-Benzylpiperidine
99%
Synonym(s):
Phenyl(4-piperidyl)methane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C12H17N
CAS Number:
Molecular Weight:
175.27
Beilstein:
132339
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
refractive index
n20/D 1.537 (lit.)
bp
279 °C (lit.)
mp
6-7 °C (lit.)
density
0.997 g/mL at 25 °C (lit.)
functional group
phenyl
SMILES string
C1CC(CCN1)Cc2ccccc2
InChI
1S/C12H17N/c1-2-4-11(5-3-1)10-12-6-8-13-9-7-12/h1-5,12-13H,6-10H2
InChI key
ABGXADJDTPFFSZ-UHFFFAOYSA-N
Gene Information
rat ... Htr2a(29595) , Htr2c(25187)
Looking for similar products? Visit Product Comparison Guide
General description
4-Benzylpiperidine is dopamine-selective releaser. It is a potential candidate for treatments for cocaine dependence.
Application
4-Benzylpiperidine was used to study the metabolism of 1,3-butadiene and its oxidized metabolites in perfused livers of male B6C3F1 mice and Sprague-Dawley rats.
Reactant for synthesis of:
- Antiproliferatives
- GABA uptake inhibitors
- Pyridines
- Histamine H3 antagonists
- Multipotent drugs with cholinergic and neuroprotective properties for the treatment of Alzheimer′s and neuronal vascular diseases
- Antiserotoninergic, antiplatelet, hemorheologic, antiarrythmic and antioxidant molecules via nucleophilic substitution
Biochem/physiol Actions
4-Benzylpiperidine inhibits the activity of rat brain monoamine oxidase-A and -B.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
233.6 °F - closed cup
Flash Point(C)
112 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Y Arai et al.
Neuroscience letters, 70(2), 255-260 (1986-10-08)
Inhibitory effects of some MPTP and MPP+ analogues on rat brain MAO activity were studied to further clarify the structure-activity relationships of MPTP neurotoxicity. Of the analogues tested, 4-(4-chlorophenyl)-1,2,3,6-tetrahydropyridine (CPTP), 4-(4-chlorobenzyl)-pyridine (CBP), 4-benzylpyridine (BPY) and 4-benzylpiperidine (BPIP) dose-dependently inhibited both
Johannes G Filser et al.
Toxicological sciences : an official journal of the Society of Toxicology, 114(1), 25-37 (2009-12-17)
The industrial chemical 1,3-butadiene (BD) is a potent carcinogen in mice and a weak one in rats. This difference is generally related to species-specific burdens by the metabolites 1,2-epoxy-3-butene (EB), 1,2:3,4-diepoxybutane (DEB), and 3,4-epoxy-1,2-butanediol (EBD), which are all formed in
Sindhu S Ganti et al.
International journal of pharmaceutics, 550(1-2), 71-78 (2018-08-21)
The objective of our study was to develop a transdermal patch of 4-benzylpiperidine and to evaluate its in vitro transdermal permeation profile. Appropriate pressure sensitive adhesives and additives were selected based on solubility and slide crystallization studies. Release liners and
S S Negus et al.
The Journal of pharmacology and experimental therapeutics, 329(1), 272-281 (2009-01-20)
Monoamine releasers constitute one class of drugs currently under investigation as potential agonist medications for the treatment of cocaine dependence. The efficacy and safety of monoamine releasers as candidate medications may be influenced in part by their relative potency to
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service