445223
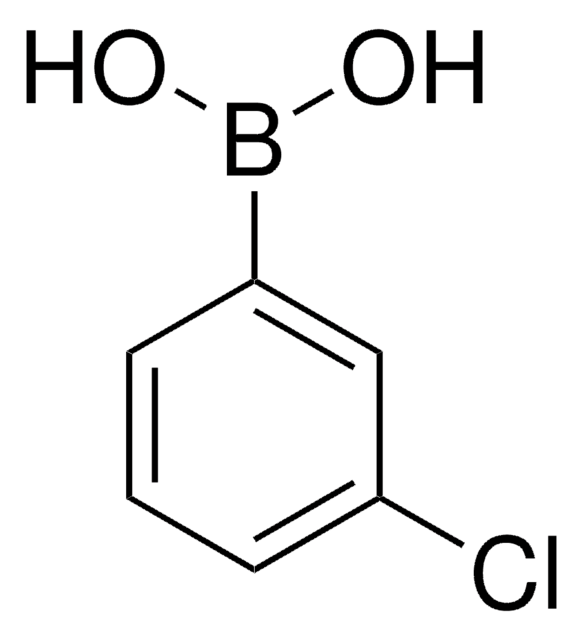
2-Fluorophenylboronic acid
≥95%
Synonym(s):
2-Fluorobenzeneboronic acid, o-fluoro-benzeneboronic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
FC6H4B(OH)2
CAS Number:
Molecular Weight:
139.92
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥95%
form
solid
mp
101-110 °C (lit.)
functional group
fluoro
SMILES string
OB(O)c1ccccc1F
InChI
1S/C6H6BFO2/c8-6-4-2-1-3-5(6)7(9)10/h1-4,9-10H
InChI key
QCSLIRFWJPOENV-UHFFFAOYSA-N
Application
Reactant for:
- Preparation of phenylboronic catechol esters as promising anion receptors for polymer electrolytes
- Diastereoselective synthesis of trisubstituted allylic alcohols via rhodium-catalyzed arylation
- Site-selective Suzuki-Miyaura arylation reactions
- Rh-catalyzed enantioselective addition reactions
- Rhodium- and Palladium-catalyzed substitution reactions
Used for the preparation of biologically active biphenyls and arylboron difluoride Lewis acids.
Other Notes
Contains varying amounts of anhydride
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Helvetica Chimica Acta, 78, 2026-2026 (1995)
The Journal of Organic Chemistry, 60, 3020-3020 (1995)
Mikhail Y Vorona et al.
Materials (Basel, Switzerland), 13(8) (2020-04-26)
Anthracene-based semiconductors have attracted great interest due to their molecular planarity, ambient and thermal stability, tunable frontier molecular orbitals and strong intermolecular interactions that can lead to good device field-effect transistor performance. In this study, we report the synthesis of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service



![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)