325228
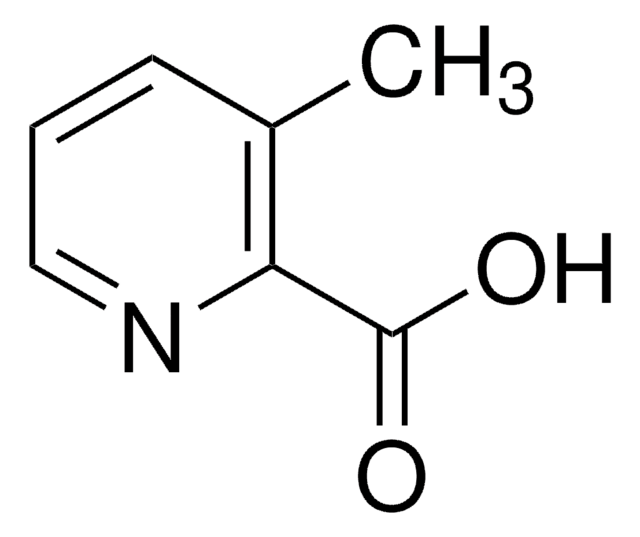
2-Methylpyridine-3-carboxylic acid
98%
Synonym(s):
2-Methylnicotinic acid, 2-Methylpyridine-3-carboxylic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C7H7NO2
CAS Number:
Molecular Weight:
137.14
Beilstein:
114333
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
228-230 °C (dec.) (lit.)
functional group
carboxylic acid
SMILES string
Cc1ncccc1C(O)=O
InChI
1S/C7H7NO2/c1-5-6(7(9)10)3-2-4-8-5/h2-4H,1H3,(H,9,10)
InChI key
HNTZKNJGAFJMHQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
2-Methylpyridine-3-carboxylic acid was used in synthesis of 1,8-dioxo-1,2,7,8-tetrahydro-2,7,10-triaza-anthracene-4,5-dicarbaldehydes (DOTTADs) and their imines. It was also used in synthesis of 7,7-dichloro-5,7-dihydro-thieno[3,4-b]pyridin-5-one.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Preparation, X-ray structure and propylaminolysis of 7, 7-dichloro-5, 7-dihydro-thieno [3, 4-b] pyridin-5-one.
van Es T, et al.
J. Chem. Res. (M), 2007(6), 373-376 (2007)
Andrea Arany et al.
Organic & biomolecular chemistry, 1(9), 1545-1551 (2003-08-21)
The interaction of Hantzsch pyridinecarboxylic acids with dialkylformamides and POCl3, followed by treatment with NH4OH yields 1,8-dioxo-1,2,7,8-tetrahydro-2,7,10-triazaanthracenes (DOTTADs), which have great potential as useful ligands for Group I and II metals and some transition metals. The corresponding Hantszch esters similarly
Ashwini Kumar Ray et al.
Molecular and biochemical parasitology, 219, 42-51 (2017-11-28)
Selective 2'-hydroxyl acylation analyzed by primer extension (SHAPE) is a versatile sequence independent method to probe RNA structure in vivo and in vitro. It has so far been tried mainly with model organisms. We show that cells of Entamoeba histolytica
Ignaz J Buerge et al.
Environmental science & technology, 53(10), 5725-5732 (2019-04-25)
Many pesticides show a pronounced biphasic degradation in soil, typically with a faster initial phase, followed by a slower decline. For chiral compounds, a biphasic decline of the total concentration may result from enantioselective degradation. In this study with the
Jamie Richards et al.
Molecular cell, 74(2), 284-295 (2019-03-11)
The diversity of mRNA lifetimes in bacterial cells is difficult to reconcile with the relaxed cleavage site specificity of RNase E, the endonuclease most important for governing mRNA degradation. This enzyme has generally been thought to locate cleavage sites by
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service